Volume 25, Issue 3 (Autumn 2024)
jrehab 2024, 25(3): 372-395 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jahantigh Akbari N, Niknam H, Sadat Naimi S, Tahan N, Danesh Shahreki B, Jahantigh Akbari A, et al . The Effect of Exercise Therapy on Muscular Strength, Bone Mineral Density and Quality of Life in Postmenopausal Women With Osteoporosis: A Systematic Review. jrehab 2024; 25 (3) :372-395
URL: http://rehabilitationj.uswr.ac.ir/article-1-3320-en.html
URL: http://rehabilitationj.uswr.ac.ir/article-1-3320-en.html
Narges Jahantigh Akbari1 

 , Hoda Niknam *2
, Hoda Niknam *2 

 , Sedigheh Sadat Naimi3
, Sedigheh Sadat Naimi3 

 , Nahid Tahan3
, Nahid Tahan3 

 , Bijan Danesh Shahreki4
, Bijan Danesh Shahreki4 

 , Ali Jahantigh Akbari5
, Ali Jahantigh Akbari5 

 , Yousef Nooshiravani6
, Yousef Nooshiravani6 

 , Negar Asad-Sajjadi7
, Negar Asad-Sajjadi7 




 , Hoda Niknam *2
, Hoda Niknam *2 

 , Sedigheh Sadat Naimi3
, Sedigheh Sadat Naimi3 

 , Nahid Tahan3
, Nahid Tahan3 

 , Bijan Danesh Shahreki4
, Bijan Danesh Shahreki4 

 , Ali Jahantigh Akbari5
, Ali Jahantigh Akbari5 

 , Yousef Nooshiravani6
, Yousef Nooshiravani6 

 , Negar Asad-Sajjadi7
, Negar Asad-Sajjadi7 


1- Department of Physical Therapy, School of Rehabilitation, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Physiotherapy Research Center, School of Rehabilitation, Shahid Beheshti University of Medical Sciences, Tehran, Iran. ,Hodaniknam@sbmu.ac.ir
3- Physiotherapy Research Center, School of Rehabilitation, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Informatics Unit, Deputy of Education, Zabol University of Medical Sciences, Zabol, Iran.
5- Department of Psychology, Faculty of Humanities, Zahedan Branch, Islamic Azad University, Zahedan, Iran.
6- Department of Biostatistics, School of Medical Sciences, Zabol University of Medical Sciences, Zabol, Iran.
7- Department of Biostatistics, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Physiotherapy Research Center, School of Rehabilitation, Shahid Beheshti University of Medical Sciences, Tehran, Iran. ,
3- Physiotherapy Research Center, School of Rehabilitation, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Informatics Unit, Deputy of Education, Zabol University of Medical Sciences, Zabol, Iran.
5- Department of Psychology, Faculty of Humanities, Zahedan Branch, Islamic Azad University, Zahedan, Iran.
6- Department of Biostatistics, School of Medical Sciences, Zabol University of Medical Sciences, Zabol, Iran.
7- Department of Biostatistics, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 2826 kb]
(1497 Downloads)
| Abstract (HTML) (6691 Views)
Full-Text: (2403 Views)
Introduction
Osteoporosis is a prevalent metabolic disorder and a substantial global health concern [1, 2]. Based on the World Health Organization (WHO), osteoporosis ranks as the third most serious global health concern after cardiovascular diseases and cancer [3]. This disease is associated with decreased bone density, weakening of the bone structure, and consequently, a reduction in bone integrity, leading to a higher probability of bone breakage [4]. This disease is common among postmenopausal women [1]. Osteoporosis resulting from menopause is significant because the early explanation of osteoporosis underscores the significance of the ovaries insufficiency and hormonal issues after menopause [5]. Menopause and subsequent estrogen reduction are considered one of the primary decisive factors of osteoporosis in women [6]. Therefore, women who have been exposed to estrogen for a shorter period during their lifetime due to reasons such as late menarche and early menopause are at a higher risk of developing osteoporosis [6].
Additionally, after menopause, the rate of bone mass decreases several times, as the decrease in ovarian activity and estrogen levels results in heightened osteoclast activity [7]. The incidence of osteoporosis and related fractures in women has been documented to be 8 times greater than in men [3]. Moreover, 60% of bone growth occurs during the adolescent period. Osteoporosis starts in adulthood and begins during growth due to inadequate bone development resources. On the other hand, in adulthood, menopause leads to a reduction in estrogen levels and bone density [3]. Therefore, during menopause and as the person ages, no correlation is observed between the quantity of bone broken down and the bone created. Hence, a defect occurs at the end of each cycle [1]. It has been noted that several factors are involved in the process of bone formation and in preventing osteoporosis. These factors include adequate nutrition containing ample protein, calcium, and vitamin D, in addition to consistent physical activity which have a significant role in maintaining bone mass, increasing muscle strength, and quality of life (QoL) improvement [8].
Bone mineral density (BMD) measurement is a criterion for osteoporosis. A decrease in T-score and more than 2 standard deviations in BMD are considered osteoporosis [9]. In this case, the individual is at the fracture threshold [9]. Osteoporosis is accompanied by various consequences, such as pain, gradual loss of height and severe kyphosis [10]. Moreover, the long-term implications of osteoporosis include falling due to balance disorders, muscle strength deterioration and fractures in different body parts, which can significantly impact the QoL for these people [11]. Moreover, regular physical activity, exercise and adequate calcium intake substantially influence upholding and improving muscle strength and bone mass by applying a load on bones and body muscles. Therefore, they help prevent fractures due to osteoporosis and falls and improve QoL [12]. Fractures resulting from osteoporosis are considered a major public health concern worldwide, with falling playing a significant role in these fractures, especially in the hip and upper limb regions [13]. Approximately 80% to 90% of hip fractures in elderly individuals occur with fallings, so in one year out of every 30 older adults, one person aged 65 and above is at risk of falling [14]. Between 50% and 70% of people who experience hip fractures do not regain their former level of functioning, and a significant number of them need extended care. Additionally, around 25% of them also become disabled and pass away in the first year following the fracture [15].
Fractures due to osteoporosis in the spine and hip bone not only lead to significant disability but also impose high treatment costs on these individuals [16]. Therefore, efforts should be made to seek treatments to preserve or improve bone density and prevent falls in these individuals [16]. Bone density improves in osteoporosis patients through pharmaceutical and non-pharmaceutical therapies [10]. Pharmaceutical treatments include taking bisphosphonates, calcium, vitamin D and hormone replacement therapy (HRT), such as progesterone, calcitonin and estrogen [17]. Pharmaceutical treatments are effective for preventing and treating this condition, but long-term use of medication has many limitations because of adverse reactions. As a result, scientists are seeking non-drug therapies [16]. Non-pharmacological treatments include physical therapy and dietary regimens [1]. People with active lifestyles have greater bone mass than individuals with lower activity levels [18]. Furthermore, regular exercise can maintain and improve bone density [1]. Moreover, based on available evidence, engaging in specific exercises may lower the chances of experiencing falls in older adulthood [19]. Sport interventions have been minimally used in women with osteoporosis [20]. The best kind of workout, its timing, and regularity have not been established yet [21]. According to meta-analysis research, aerobic and resistance exercises are associated with improved bone density compared to the healthy group [22]. Therefore, in osteoporotic individuals, regular physical activity can help lower the chances of experiencing fractures by keeping bone mass and improving stability and balance, thus minimizing the rate of falls [23].
Carter et al. performed a randomized controlled trial (RCT) to examine exercise therapy in osteoporotic postmenopausal women aged between 65 and 75 years. The findings of this study demonstrated that individuals in the exercise therapy group experienced improvements in muscle strength, which is a crucial subject in determining falling in older osteoporotic women [1]. In another study, Iwamoto et al. compared three types of endurance exercise intensities. This research demonstrated that a 12 m/min training plan for 1 hour per day improved the tibia bone’s mass and the femur’s strength. Interestingly, The bone mass of the fifth lumbar vertebra did not show a significant difference in any exercise group when compared to the healthy group [24]. Arnold et al. compared water-based exercises with land-based ones on QoL and function in elderly osteoporotic women. The performance and QoL did not significantly differ between women who participated in water-based exercises and those who engaged in land-based exercises, compared to the control group [13]. Hourigan et al. conducted a study involving 50 osteoporotic patients who received regular exercise sessions twice weekly for 2 weeks. This research demonstrated that balance training and core muscle strengthening exercises improved balance, reduced fallings, and increased muscle strength in osteoporotic women [25]. Moreover, it has been reported that weight-bearing activities like running, walking, dancing, and jumping result in the generation of muscular contractions and improvement in muscle performance [26].
Nowadays, with increasing hope for life and the growth of the elderly population, the prevalence of this disease is on the rise [27]. In addition, because a considerable portion of the female population lacks sufficient physical activity, they are more susceptible to osteoporosis [28]. Furthermore, the potential long-term effects of osteoporosis, like higher rates of bone fractures and physical changes like kyphosis and pain, have been shown to impact the QoL [29]. Therefore, with the increasing prevalence of this disease, disability, mortality, and healthcare costs will increase [30]. A notable correlation exists between bone mass and physical activity [28]. Considering these factors and the significance of physical activity treatment in these individuals, a review study in this field will help determine the effective exercise type for these patients. Therefore, this systematic review research aimed to examine the effectiveness of exercise therapy on muscle strength, bone mass and QoL in osteoporotic postmenopausal women.
Materials and Methods
This review study followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist. This guideline aims to assist authors in developing a meta-analysis or systematic review. The guideline provides a comprehensive method for reporting systematic review studies [31].
Eligibility criteria
The accepted studies met the following criteria for study inclusion:
1) The design had to be RCT; 2) Participants were women with osteoporosis; 3) Only exercise-based interventions were investigated. Therefore, if exercise-based interventions were used along with other treatments, they were excluded from the review process; 4) Studies had to include a control group that does not engage in physical activity or receive exercise treatment; 5) Variables must encompass muscle strength, bone mass, and QoL; 6) Studies must have examined menopausal women; 7) The study must have been conducted on human samples; 8) Studies should provide tools for measuring variables included in this research; 9) Studies conducted in the English language have been reviewed.
Studies were excluded that met the following criteria:
1) If participants were not suffering from osteoporosis; 2) If the intervention was other than exercise therapy; 3) Variables other than bone density, muscle strength, and QoL; 4) Studies that did not include postmenopausal women; 5) Studies that did not involve human samples; 6) Studies that had qualitative measurements.
Information sources
To find the desired articles on the impact of exercise therapy on bone mass, QoL and muscle strength in osteoporotic postmenopausal women, we examined the related articles for entering a systematic review study between 1996 and 2022. Databases like Cochrane, PubMed, Google Scholar and ScienceDirect were used for searching, and the search was conducted in December 2022.
Search strategy
Both MeSH terms and keywords were utilized in all electronic databases. This combination included “exercise therapy” OR “ physical activity” OR “exercise” OR “physical therapy” AND “bone mass” OR “bone mineral density” AND “muscle strength” OR “strength training” OR “strengthening” AND “quality of life,” AND “osteoporosis,” AND “menopausal women.”
Study selection
Upon obtaining the complete texts of the chosen articles for the ultimate assessment, two authors independently reviewed them for the inclusion and exclusion criteria. Articles that met the criteria for inclusion were selected to be included in the study.
Quality assessment
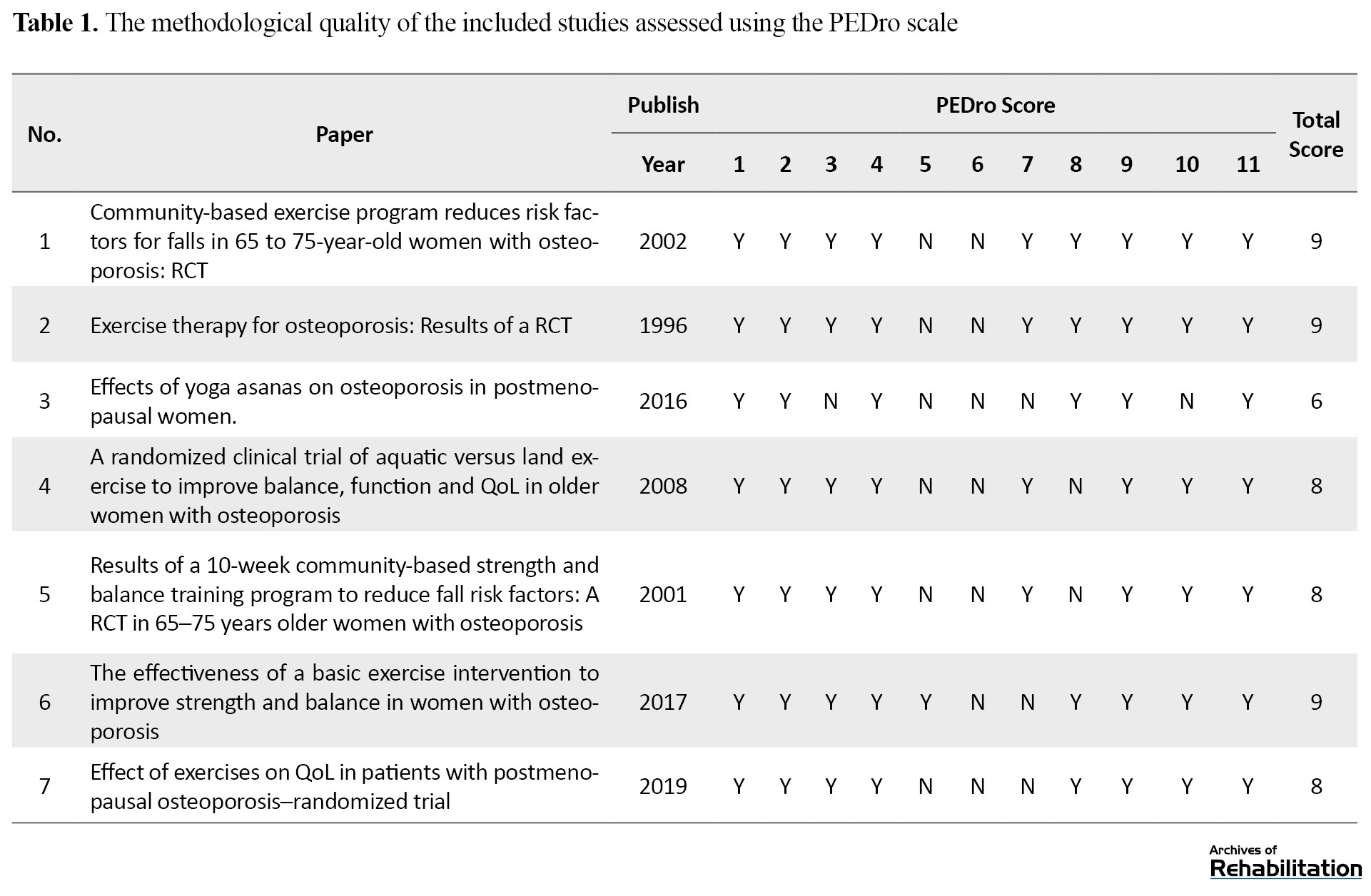
The methodological quality of each article was assessed using the Physiotherapy Evidence Database (PEDro) scale. Any discrepancies between the two authors were resolved through agreement. This scale comprises 11 criteria utilized to evaluate the quality of RCT studies in physiotherapy. PEDro has the reliability and necessary validity for evaluating RCT studies. There are 11 two-part items with “yes” and “no” responses. “Yes” indicates a score of 1, and “no” means 0. Higher scores indicate higher quality of the selected articles (Table 1) [32].
Data collection
Estimating each variable’s effect size, such as bone density, muscle strength, and QoL, involves calculating the mean and standard deviation from eligible articles that the two authors performed. If the article information was unavailable, the corresponding author contacted the author of the specific article.
Measurement of the variables
Several different assessment tools for measuring muscle strength, bone density, and QoL were utilized in seven selected studies for the final evaluation. The dynamometer test, arm curl test, and 30-s chair test were used in three studies to evaluate muscle strength [1, 33, 34], dual-energy x-ray absorptiometry (DEXA) in two articles for assessing bone mass [35, 36], and the OP QOL and Qualeffo-41 questionnaires were also used in 2 articles to determine the QoL [13, 37].
Data items
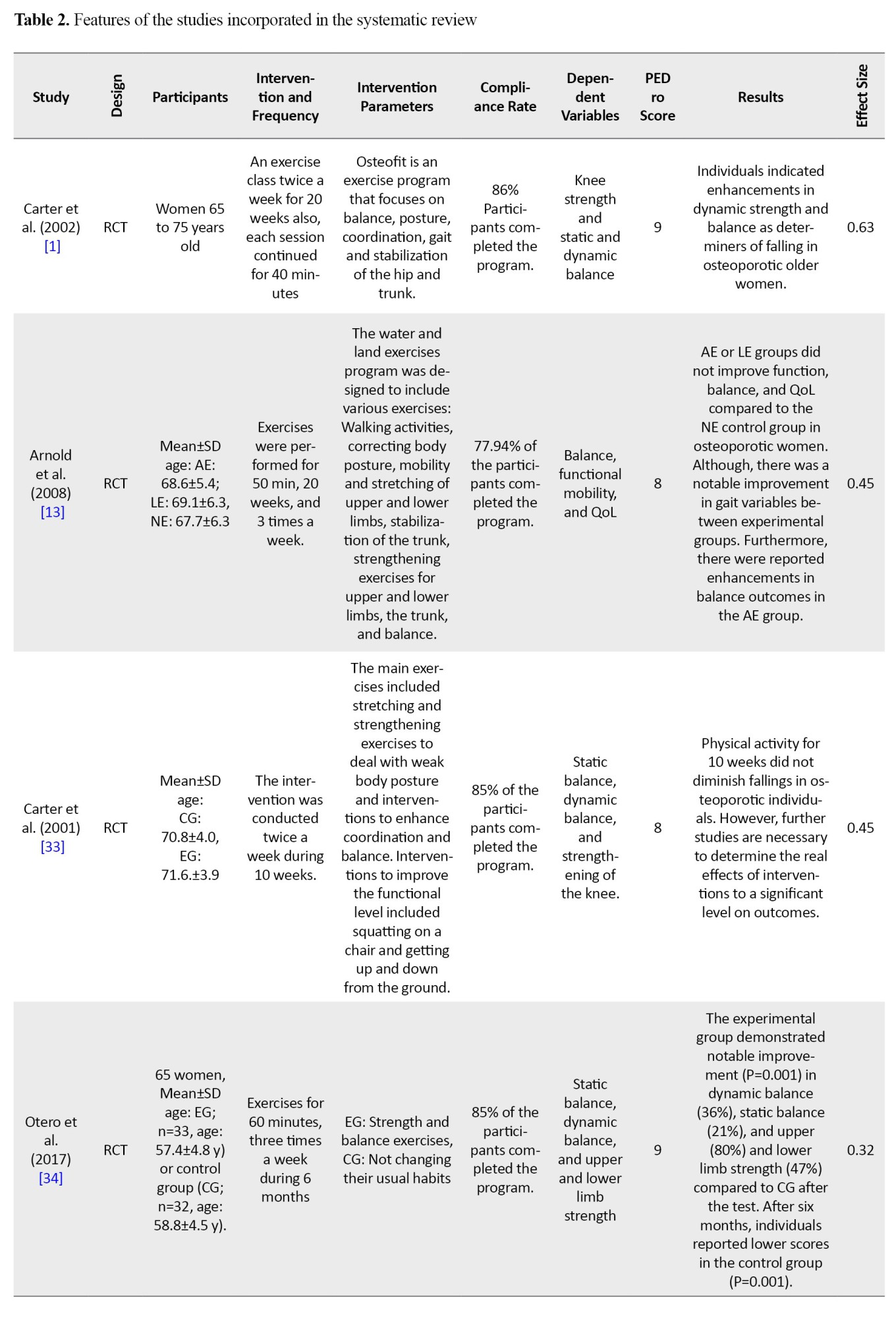
The subsequent data was derived from the articles: 1) participant traits (comprising gender, age, and diagnosis of osteoporosis), 2) intervention type and the number of individuals in the control and intervention groups, 3) the type of measurement tool used for each variable and the duration during the period from the beginning of the treatment to the follow-up, and 4) the average and standard deviation for the start of treatment and follow-up for each variable were extracted (Table 2).

Measurement summary
In the current systematic review study, the measurement of Cohen’s effect size has been used to examine the effect of treatment on targeted outcomes. The effect size measurement was conducted for the target variable, considering the average and standard deviation at the initiation of therapy and follow-up. Cohen’s d is described as an effect size measure, considering 0.0-0.10 as negligible, 0.20 to 0.40 as small, 0.50 to 0.70 as moderate, and over 0.80 as large.
Results
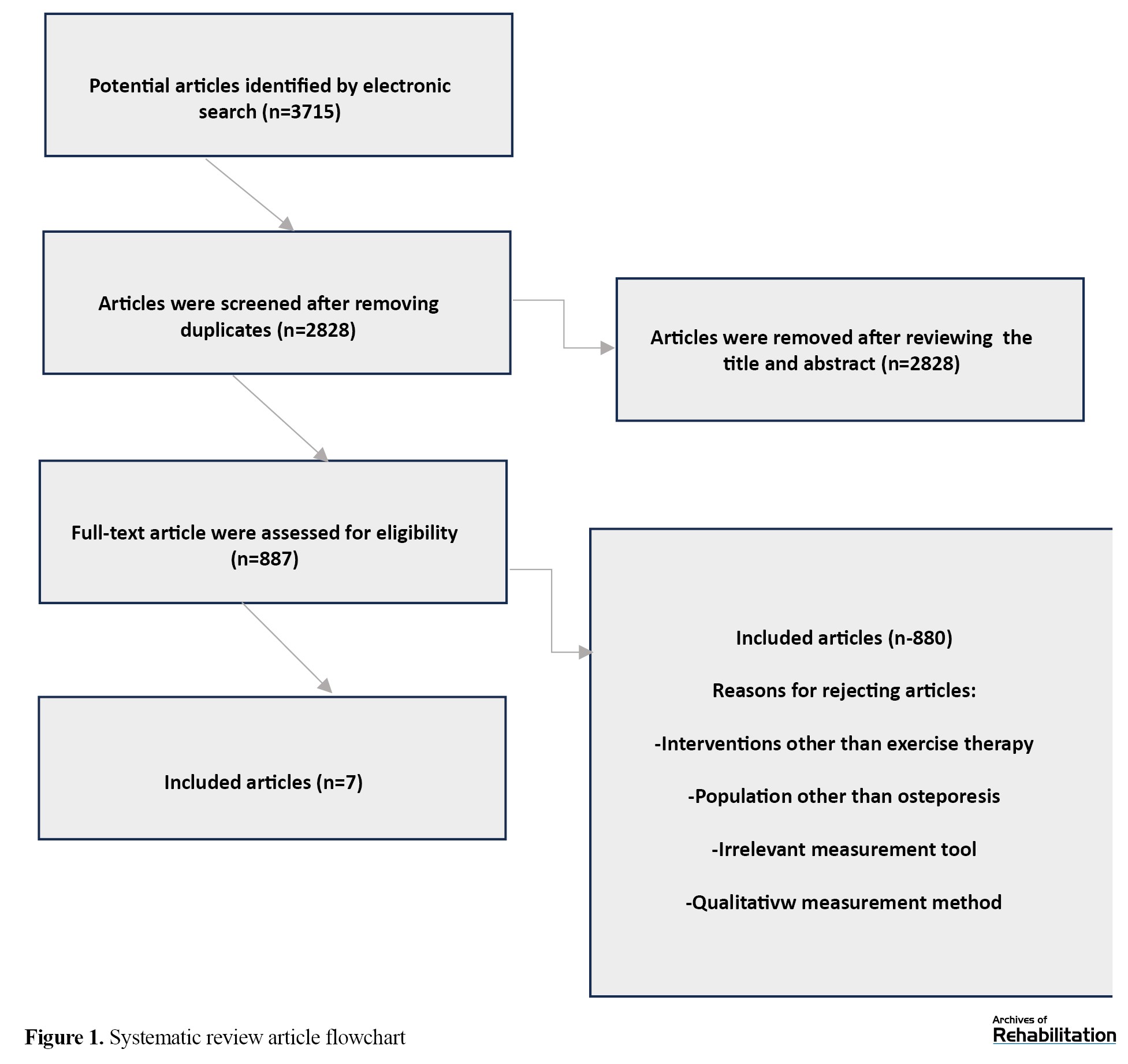
In total, 3715 relevant articles were found in the initial search, of which 887 had complete texts. Two authors reviewed these articles. Out of these 887 articles, most were removed due to irrelevant Titles, Abstracts, duplicate content, and other factors (such as review articles, non-RCT articles, and those unrelated to the analysis). Moreover, articles with interventions other than exercise therapy or those whose study participants did not consist of postmenopausal women with osteoporosis were excluded. Furthermore, articles with healthy individuals in their control group were excluded from the list of qualifying articles. In the end, 8 articles were chosen for the final assessment. Out of these, one article was eliminated from the study due to inadequate use of the therapeutic intervention. Thus, only 7 articles met the inclusion criteria (Figure 1). The sample size varied between 30 to 92 participants in 7 studies, with total estimated participants of 506 across the studies. Studies meeting the criteria were rated between 6 and 9 on the PEDro scale. Seven qualifying studies utilized Various measurement instruments to evaluate QoL, muscle strength, and bone density. Muscle strength was examined through dynamometer test, 30-s chair stand test, and arm curl in three studies [1, 33, 34], DEXA was employed in two articles to measure bone density [35, 36], OP QOL and Qualeffo-41 questionnaires were utilized in 2 eligible articles to assess QoL [13, 37]. The intervention effect size was measured between 0.32 (small) to 0.63 (moderate). Five studies indicated a small effect size, while 2 studies showed a moderate effect size. The study results suggested that exercises focusing on balance, strength, resistance, stability and motor control impacted muscle strength and bone mass in osteoporotic postmenopausal women.
Discussion
Osteoporosis is a significant health concern. This condition leads to decreased bone density and a decline in overall well-being, imposing considerable financial burdens on patients who experience fractures [38]. The significance of this condition lies in the increased risk of fractures, including the femur, spinal column, and hip [39]. This issue has resulted in a rise in mortality rates and medical expenses [40]. Furthermore, menopause between the ages of 40 and 45, a brief fertility window, reduced bone density during skeletal maturation, and rapid bone mass loss post-menopause are associated with increasing the likelihood of osteoporosis in older women [5]. Hence, women in menopause with these risk factors should take into account osteoporosis seriously [5]. Moreover, reports indicate that the likelihood of bone fractures resulting from osteoporosis among postmenopausal women is 46% in Sweden and 40% in the United States [41]. These statistics underscore the significance of managing osteoporosis in women who have undergone menopause. Hence, this systematic review research aimed to investigate the impact of exercise therapy on bone density, muscle strength, and QoL in women who have undergone menopause and have osteoporosis.
In this review, 7 studies were chosen for final assessment. The studies were assessed for their methodological quality using the PEDro scale. The evaluation resulted in quality scores between 6 and 9 for these studies, demonstrating their high methodological quality. Cohen’s d was also used to assess the effect size of exercise therapy interventions. According to these findings, exercise therapy interventions in patients with osteoporosis significantly improved satisfaction. Five studies revealed a minor effect size, 2 studies had a minor effect size on muscle strength, one study showed a minor effect size on bone density and 2 studies reported a minor effect size on the QoL [13, 33, 34, 35, 37]. However, other studies documented a moderate effect size on muscle strength and bone density [1, 36]. The findings of this study revealed the impact of balance, strength, flexibility, stability, and motor control exercises on muscle strength and bone mass in osteoporotic postmenopausal women. Variations in the effect size among studies are influenced by different characteristics such as treatment duration, follow-up period, frequency, intensity, and treatment approach. Some studies have a follow-up period of 4 years, while others are as short as 6 months. However, some studies have a short follow-up period.
The utilized exercise interventions encompass strength, balance, flexibility, postural stability, movement control, muscle strength, yoga, aquatic and land exercises [1, 13, 33, 35, 36]. Also, the variability in treatment modalities, encompassing exercise duration and volume, was highlighted. Five studies examined the impacts of yoga and aquatic exercises versus exercises done on land, strength exercises, breathing exercises, range of motion, and balance exercises over periods of 3, 10, 20, 24 weeks, and 6 months, with sessions lasting 50 to 60 minutes [13, 33, 34, 35, 37], despite the studies showing small effect sizes [13, 33-35, 37]. The effect sizes were moderate in two additional studies that focused on investigating the combined effects of strength and balance training. These activities were carried out for 40 min during each session over 20 weeks [1, 36]. In one of these two studies, the variable under investigation was muscular strength, measured using a dynamometer. In the other study, the variable examined was bone density, evaluated using single-photon absorptiometry [1, 36].
Three RCT studies indicated a notable improvement in the strengthening of muscles following the implementation of balance, resistance, and flexibility exercises [1, 33, 34]. Two RCT studies focused on investigating the impact of yoga, stretching, movement control exercises, and stability exercises on bone density in osteoporotic patients, with these exercises showing a significant improvement in this variable [35, 36]. Two other RCT studies examined the effects of respiratory, strengthening, balance, range of motion, aerobic, and aquatic exercises compared to land exercises on QoL [13, 37]. A study indicated a noteworthy enhancement in the QoL [37]. Meanwhile, a different study noted a considerable enhancement within every intervention group, including water versus land exercises and the control group; no notable disparity was observed between groups [13].
Muscle strength
In individuals with osteoporosis, the main reason for fractures has been reported to be falling due to impaired balance and reduced muscle strength [42], highlighting the importance of possessing strong lower limb muscles and sustaining a stable balance as crucial factors in independence and the capability to participate in everyday tasks [43]. Hence, enhancing muscle strength and balance can be attained through engaging in sports activities, which has been demonstrated in various studies [44]. In this review study, three studies investigated the impacts of different training on muscle strength in osteoporotic patients. These studies reported a notable increase in muscle power following exercise therapy, which was aligned with the results of the existing review study [1, 33, 34]. One study had a medium effect size [1], while two others had small effect sizes [33, 34]. Exercise can improve muscle strength, balance, postural stability, range of motion, muscle endurance and flexibility by enhancing neuromuscular coordination. Consequently, the incidence of falls and their fractures is reduced in these individuals, improving their QoL [45]. Moreover, improvement in muscle strength in these studies may be linked to the duration of performing these exercises. In other words, consistent and regular exercises enhanced muscle strength in the group receiving exercise therapy as opposed to the control group. In contrast, discontinuing exercise led to a decline in the benefits of the workout. Also, the studies involved a combination of exercises and these combined exercises with effective intensity played an optimal role in increasing muscle strength compared to each exercise separately [46].
Bone density
Tiny structures (organizational spatial structure of bone tissue) and bone mass are important determinants of bone mechanical capability against fractures. It is essential to examine these factors when assessing the impact of aging on bone tissue [47]. With aging and the deterioration of tiny structures, bone fragility occurs in both genders with unequal ratios [47]. Therefore, it is necessary to use treatments to preserve the microscopic structures and improve bone density. This review article includes two studies investigating how exercise therapy affects bone density in osteoporotic individuals [35]. One study with a small effect size [35] and another with a moderate effect size reported a significant improvement aligned with the existing research results [36]. Concerning the interpretation of the effect size categorized as “moderate” in the study by Preisinger et al. which demonstrated a moderate effect size of 0.63, it can be said that in this study, the frequency, duration of exercise, and patient’s adherence to the intervention had a positive impact on the variables. In other words, all participants completed the treatment in this study, which could have played a role in increasing the effect size [36]. Therefore, the impact of exercise therapy interventions cannot be determined without considering the patients’ follow-up rate [48, 49, 50].
Physical activity is beneficial for enhancing bone density and decreasing the likelihood of fractures in osteoporotic individuals through its impact on bone characteristics [51]. The mechanical load applied to bone tissue within the lacunar-canalicular network creates a guardian that plays a role in events that occur within cells, such as heightened levels of internal calcium, matrix creation, osteogenesis, and ultimately contributing to improved bone density [48]. It has also been shown that strength training contributes to changes in bone structure by creating and distributing non-uniform mechanical pressures [52]. In this type of exercise, optimizing mechanical load and high levels of strain leads to improved bone density [53].
QoL
Osteoporosis is a complex condition that affects many facets of a person’s activity [54]. Various studies reported a significant relationship between osteoporosis and a decrease in the QoL for the individual [54]. Decreased QoL caused by osteoporosis is primarily linked to discomfort and limitations in physical abilities resulting from fractures as a consequence of the disease, as well as physical and social impairments in women following vertebral fractures [54]. On the other hand, a decline in overall well-being can stem from the fear of potential fractures that may occur in the future for the individual, or the individual may feel the need to modify their way of life to avoid possible fractures that may occur in the future [55]. In this review study, 2 studies investigated how different exercises impact the QoL in osteoporotic individuals [13, 37]. Both studies reported a small effect size and a significant improvement that aligns with the findings of the existing review research [13, 37].
Reports have shown that exercise therapy and home training can effectively enhance the QoL. This improvement in the QoL has resulted in a decrease in the likelihood of experiencing falls by up to 50% [56]. Moreover, the positive impact of exercise and group activities on mental health indicators and psychosomatic symptoms can improve motivation and encourage patients to participate in exercise programs. Therefore, increasing patients’ physical activity enhances their daily activities and improves their QoL [57]. It has been reported that compared to other interventions, exercise therapy has positive and significant effects in osteoporotic postmenopausal women. Fishman et al. showed a positive change in spine and hip T-score index following a 2-year pilot study. On the other hand, this study reported a greater enhancement in pelvic bone density than in the spine [58]. Soomro et al. examined the impact of physical activity on preventing osteoporosis compared with walking in young women. They did not report a difference in T-score between the two groups during 3 months. Therefore, to investigate the effect of exercise therapy, it is necessary to conduct more studies over a more extended period [59].
We only included randomized clinical trials to assess the quality of studies better. Most research demonstrated moderately high methodological standards and low and moderate effect sizes. Therefore, effect size measurement can ascertain the impacts of exercise treatment. Based on the results of the present review, physiotherapists and other healthcare professionals can expect many benefits following exercise treatment in osteoporotic individuals.
There were limitations in the present systematic review. First, we only considered studies published in English. Second, all the studies in the present study had a PEDro score between 6 and 9, of medium to high quality. The PEDro scale score in the range of more than 5 is a medium to high score. Therefore, in this case, there is a risk regarding the effects related to the quality of the studies. Third, the studies selected for this review had different follow-up periods. Therefore, it is suggested that the evaluated studies should be similar regarding the follow-up and research periods to make comparing the results easier. In addition, most studies had a brief duration of follow-up. Therefore, it is preferable to consider studies with an extended follow-up period. It is also recommended that studies with more participants be selected for final evaluation.
Conclusion
This research demonstrated the impacts of balance, strength, stretching, stability, and movement control exercises on bone mass and muscle strength in osteoporotic postmenopausal women. However, more studies are needed in this field.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.RETECH.REC.1399.1001).
Funding
This research was supported by a research project (No.: 63675 /1399), Funded by Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors' contributions
Conceptualization: Narges Jahantigh Akbari, Hoda Niknam, Sedigheh Sadat Naimi, Bijan Danesh Shahreki, Ali Jahantigh Akbari and Yousef Nooshiravani; Methodology: Narges Jahantigh Akbari, Hoda Niknam, Bijan Danesh Shahreki, Ali Jahantigh Akbari, Nahid Tahan, and Sedigheh Sadat Naimi; Research, review, analysis and drafting: Narges Jahantigh Akbari, Hoda Niknam, Sedigheh Sadat Naimi, Bijan Danesh Shahreki and Negar Asad-Sajjadi; Sources: Narges Jahantigh Akbari and Hoda Niknam; Visualization: Narges Jahantigh Akbari and Hoda Niknam; Supervision: Narges Jahantigh Akbari, Hoda Niknam, and Sedigheh Sadat Naimi; Project management: Narges Jahantigh Akbari, Hoda Niknam, and Bijan Danesh Shahreki; Review, editing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate the Student Research Committee and Head of Research and Technology at Shahid Beheshti University of Medical Sciences for their financial support of this study.
References
Osteoporosis is a prevalent metabolic disorder and a substantial global health concern [1, 2]. Based on the World Health Organization (WHO), osteoporosis ranks as the third most serious global health concern after cardiovascular diseases and cancer [3]. This disease is associated with decreased bone density, weakening of the bone structure, and consequently, a reduction in bone integrity, leading to a higher probability of bone breakage [4]. This disease is common among postmenopausal women [1]. Osteoporosis resulting from menopause is significant because the early explanation of osteoporosis underscores the significance of the ovaries insufficiency and hormonal issues after menopause [5]. Menopause and subsequent estrogen reduction are considered one of the primary decisive factors of osteoporosis in women [6]. Therefore, women who have been exposed to estrogen for a shorter period during their lifetime due to reasons such as late menarche and early menopause are at a higher risk of developing osteoporosis [6].
Additionally, after menopause, the rate of bone mass decreases several times, as the decrease in ovarian activity and estrogen levels results in heightened osteoclast activity [7]. The incidence of osteoporosis and related fractures in women has been documented to be 8 times greater than in men [3]. Moreover, 60% of bone growth occurs during the adolescent period. Osteoporosis starts in adulthood and begins during growth due to inadequate bone development resources. On the other hand, in adulthood, menopause leads to a reduction in estrogen levels and bone density [3]. Therefore, during menopause and as the person ages, no correlation is observed between the quantity of bone broken down and the bone created. Hence, a defect occurs at the end of each cycle [1]. It has been noted that several factors are involved in the process of bone formation and in preventing osteoporosis. These factors include adequate nutrition containing ample protein, calcium, and vitamin D, in addition to consistent physical activity which have a significant role in maintaining bone mass, increasing muscle strength, and quality of life (QoL) improvement [8].
Bone mineral density (BMD) measurement is a criterion for osteoporosis. A decrease in T-score and more than 2 standard deviations in BMD are considered osteoporosis [9]. In this case, the individual is at the fracture threshold [9]. Osteoporosis is accompanied by various consequences, such as pain, gradual loss of height and severe kyphosis [10]. Moreover, the long-term implications of osteoporosis include falling due to balance disorders, muscle strength deterioration and fractures in different body parts, which can significantly impact the QoL for these people [11]. Moreover, regular physical activity, exercise and adequate calcium intake substantially influence upholding and improving muscle strength and bone mass by applying a load on bones and body muscles. Therefore, they help prevent fractures due to osteoporosis and falls and improve QoL [12]. Fractures resulting from osteoporosis are considered a major public health concern worldwide, with falling playing a significant role in these fractures, especially in the hip and upper limb regions [13]. Approximately 80% to 90% of hip fractures in elderly individuals occur with fallings, so in one year out of every 30 older adults, one person aged 65 and above is at risk of falling [14]. Between 50% and 70% of people who experience hip fractures do not regain their former level of functioning, and a significant number of them need extended care. Additionally, around 25% of them also become disabled and pass away in the first year following the fracture [15].
Fractures due to osteoporosis in the spine and hip bone not only lead to significant disability but also impose high treatment costs on these individuals [16]. Therefore, efforts should be made to seek treatments to preserve or improve bone density and prevent falls in these individuals [16]. Bone density improves in osteoporosis patients through pharmaceutical and non-pharmaceutical therapies [10]. Pharmaceutical treatments include taking bisphosphonates, calcium, vitamin D and hormone replacement therapy (HRT), such as progesterone, calcitonin and estrogen [17]. Pharmaceutical treatments are effective for preventing and treating this condition, but long-term use of medication has many limitations because of adverse reactions. As a result, scientists are seeking non-drug therapies [16]. Non-pharmacological treatments include physical therapy and dietary regimens [1]. People with active lifestyles have greater bone mass than individuals with lower activity levels [18]. Furthermore, regular exercise can maintain and improve bone density [1]. Moreover, based on available evidence, engaging in specific exercises may lower the chances of experiencing falls in older adulthood [19]. Sport interventions have been minimally used in women with osteoporosis [20]. The best kind of workout, its timing, and regularity have not been established yet [21]. According to meta-analysis research, aerobic and resistance exercises are associated with improved bone density compared to the healthy group [22]. Therefore, in osteoporotic individuals, regular physical activity can help lower the chances of experiencing fractures by keeping bone mass and improving stability and balance, thus minimizing the rate of falls [23].
Carter et al. performed a randomized controlled trial (RCT) to examine exercise therapy in osteoporotic postmenopausal women aged between 65 and 75 years. The findings of this study demonstrated that individuals in the exercise therapy group experienced improvements in muscle strength, which is a crucial subject in determining falling in older osteoporotic women [1]. In another study, Iwamoto et al. compared three types of endurance exercise intensities. This research demonstrated that a 12 m/min training plan for 1 hour per day improved the tibia bone’s mass and the femur’s strength. Interestingly, The bone mass of the fifth lumbar vertebra did not show a significant difference in any exercise group when compared to the healthy group [24]. Arnold et al. compared water-based exercises with land-based ones on QoL and function in elderly osteoporotic women. The performance and QoL did not significantly differ between women who participated in water-based exercises and those who engaged in land-based exercises, compared to the control group [13]. Hourigan et al. conducted a study involving 50 osteoporotic patients who received regular exercise sessions twice weekly for 2 weeks. This research demonstrated that balance training and core muscle strengthening exercises improved balance, reduced fallings, and increased muscle strength in osteoporotic women [25]. Moreover, it has been reported that weight-bearing activities like running, walking, dancing, and jumping result in the generation of muscular contractions and improvement in muscle performance [26].
Nowadays, with increasing hope for life and the growth of the elderly population, the prevalence of this disease is on the rise [27]. In addition, because a considerable portion of the female population lacks sufficient physical activity, they are more susceptible to osteoporosis [28]. Furthermore, the potential long-term effects of osteoporosis, like higher rates of bone fractures and physical changes like kyphosis and pain, have been shown to impact the QoL [29]. Therefore, with the increasing prevalence of this disease, disability, mortality, and healthcare costs will increase [30]. A notable correlation exists between bone mass and physical activity [28]. Considering these factors and the significance of physical activity treatment in these individuals, a review study in this field will help determine the effective exercise type for these patients. Therefore, this systematic review research aimed to examine the effectiveness of exercise therapy on muscle strength, bone mass and QoL in osteoporotic postmenopausal women.
Materials and Methods
This review study followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist. This guideline aims to assist authors in developing a meta-analysis or systematic review. The guideline provides a comprehensive method for reporting systematic review studies [31].
Eligibility criteria
The accepted studies met the following criteria for study inclusion:
1) The design had to be RCT; 2) Participants were women with osteoporosis; 3) Only exercise-based interventions were investigated. Therefore, if exercise-based interventions were used along with other treatments, they were excluded from the review process; 4) Studies had to include a control group that does not engage in physical activity or receive exercise treatment; 5) Variables must encompass muscle strength, bone mass, and QoL; 6) Studies must have examined menopausal women; 7) The study must have been conducted on human samples; 8) Studies should provide tools for measuring variables included in this research; 9) Studies conducted in the English language have been reviewed.
Studies were excluded that met the following criteria:
1) If participants were not suffering from osteoporosis; 2) If the intervention was other than exercise therapy; 3) Variables other than bone density, muscle strength, and QoL; 4) Studies that did not include postmenopausal women; 5) Studies that did not involve human samples; 6) Studies that had qualitative measurements.
Information sources
To find the desired articles on the impact of exercise therapy on bone mass, QoL and muscle strength in osteoporotic postmenopausal women, we examined the related articles for entering a systematic review study between 1996 and 2022. Databases like Cochrane, PubMed, Google Scholar and ScienceDirect were used for searching, and the search was conducted in December 2022.
Search strategy
Both MeSH terms and keywords were utilized in all electronic databases. This combination included “exercise therapy” OR “ physical activity” OR “exercise” OR “physical therapy” AND “bone mass” OR “bone mineral density” AND “muscle strength” OR “strength training” OR “strengthening” AND “quality of life,” AND “osteoporosis,” AND “menopausal women.”
Study selection
Upon obtaining the complete texts of the chosen articles for the ultimate assessment, two authors independently reviewed them for the inclusion and exclusion criteria. Articles that met the criteria for inclusion were selected to be included in the study.
Quality assessment
The methodological quality of each article was assessed using the Physiotherapy Evidence Database (PEDro) scale. Any discrepancies between the two authors were resolved through agreement. This scale comprises 11 criteria utilized to evaluate the quality of RCT studies in physiotherapy. PEDro has the reliability and necessary validity for evaluating RCT studies. There are 11 two-part items with “yes” and “no” responses. “Yes” indicates a score of 1, and “no” means 0. Higher scores indicate higher quality of the selected articles (Table 1) [32].

Data collection
Estimating each variable’s effect size, such as bone density, muscle strength, and QoL, involves calculating the mean and standard deviation from eligible articles that the two authors performed. If the article information was unavailable, the corresponding author contacted the author of the specific article.
Measurement of the variables
Several different assessment tools for measuring muscle strength, bone density, and QoL were utilized in seven selected studies for the final evaluation. The dynamometer test, arm curl test, and 30-s chair test were used in three studies to evaluate muscle strength [1, 33, 34], dual-energy x-ray absorptiometry (DEXA) in two articles for assessing bone mass [35, 36], and the OP QOL and Qualeffo-41 questionnaires were also used in 2 articles to determine the QoL [13, 37].
Data items
The subsequent data was derived from the articles: 1) participant traits (comprising gender, age, and diagnosis of osteoporosis), 2) intervention type and the number of individuals in the control and intervention groups, 3) the type of measurement tool used for each variable and the duration during the period from the beginning of the treatment to the follow-up, and 4) the average and standard deviation for the start of treatment and follow-up for each variable were extracted (Table 2).


Measurement summary
In the current systematic review study, the measurement of Cohen’s effect size has been used to examine the effect of treatment on targeted outcomes. The effect size measurement was conducted for the target variable, considering the average and standard deviation at the initiation of therapy and follow-up. Cohen’s d is described as an effect size measure, considering 0.0-0.10 as negligible, 0.20 to 0.40 as small, 0.50 to 0.70 as moderate, and over 0.80 as large.
Results
In total, 3715 relevant articles were found in the initial search, of which 887 had complete texts. Two authors reviewed these articles. Out of these 887 articles, most were removed due to irrelevant Titles, Abstracts, duplicate content, and other factors (such as review articles, non-RCT articles, and those unrelated to the analysis). Moreover, articles with interventions other than exercise therapy or those whose study participants did not consist of postmenopausal women with osteoporosis were excluded. Furthermore, articles with healthy individuals in their control group were excluded from the list of qualifying articles. In the end, 8 articles were chosen for the final assessment. Out of these, one article was eliminated from the study due to inadequate use of the therapeutic intervention. Thus, only 7 articles met the inclusion criteria (Figure 1). The sample size varied between 30 to 92 participants in 7 studies, with total estimated participants of 506 across the studies. Studies meeting the criteria were rated between 6 and 9 on the PEDro scale. Seven qualifying studies utilized Various measurement instruments to evaluate QoL, muscle strength, and bone density. Muscle strength was examined through dynamometer test, 30-s chair stand test, and arm curl in three studies [1, 33, 34], DEXA was employed in two articles to measure bone density [35, 36], OP QOL and Qualeffo-41 questionnaires were utilized in 2 eligible articles to assess QoL [13, 37]. The intervention effect size was measured between 0.32 (small) to 0.63 (moderate). Five studies indicated a small effect size, while 2 studies showed a moderate effect size. The study results suggested that exercises focusing on balance, strength, resistance, stability and motor control impacted muscle strength and bone mass in osteoporotic postmenopausal women.
Discussion
Osteoporosis is a significant health concern. This condition leads to decreased bone density and a decline in overall well-being, imposing considerable financial burdens on patients who experience fractures [38]. The significance of this condition lies in the increased risk of fractures, including the femur, spinal column, and hip [39]. This issue has resulted in a rise in mortality rates and medical expenses [40]. Furthermore, menopause between the ages of 40 and 45, a brief fertility window, reduced bone density during skeletal maturation, and rapid bone mass loss post-menopause are associated with increasing the likelihood of osteoporosis in older women [5]. Hence, women in menopause with these risk factors should take into account osteoporosis seriously [5]. Moreover, reports indicate that the likelihood of bone fractures resulting from osteoporosis among postmenopausal women is 46% in Sweden and 40% in the United States [41]. These statistics underscore the significance of managing osteoporosis in women who have undergone menopause. Hence, this systematic review research aimed to investigate the impact of exercise therapy on bone density, muscle strength, and QoL in women who have undergone menopause and have osteoporosis.
In this review, 7 studies were chosen for final assessment. The studies were assessed for their methodological quality using the PEDro scale. The evaluation resulted in quality scores between 6 and 9 for these studies, demonstrating their high methodological quality. Cohen’s d was also used to assess the effect size of exercise therapy interventions. According to these findings, exercise therapy interventions in patients with osteoporosis significantly improved satisfaction. Five studies revealed a minor effect size, 2 studies had a minor effect size on muscle strength, one study showed a minor effect size on bone density and 2 studies reported a minor effect size on the QoL [13, 33, 34, 35, 37]. However, other studies documented a moderate effect size on muscle strength and bone density [1, 36]. The findings of this study revealed the impact of balance, strength, flexibility, stability, and motor control exercises on muscle strength and bone mass in osteoporotic postmenopausal women. Variations in the effect size among studies are influenced by different characteristics such as treatment duration, follow-up period, frequency, intensity, and treatment approach. Some studies have a follow-up period of 4 years, while others are as short as 6 months. However, some studies have a short follow-up period.
The utilized exercise interventions encompass strength, balance, flexibility, postural stability, movement control, muscle strength, yoga, aquatic and land exercises [1, 13, 33, 35, 36]. Also, the variability in treatment modalities, encompassing exercise duration and volume, was highlighted. Five studies examined the impacts of yoga and aquatic exercises versus exercises done on land, strength exercises, breathing exercises, range of motion, and balance exercises over periods of 3, 10, 20, 24 weeks, and 6 months, with sessions lasting 50 to 60 minutes [13, 33, 34, 35, 37], despite the studies showing small effect sizes [13, 33-35, 37]. The effect sizes were moderate in two additional studies that focused on investigating the combined effects of strength and balance training. These activities were carried out for 40 min during each session over 20 weeks [1, 36]. In one of these two studies, the variable under investigation was muscular strength, measured using a dynamometer. In the other study, the variable examined was bone density, evaluated using single-photon absorptiometry [1, 36].
Three RCT studies indicated a notable improvement in the strengthening of muscles following the implementation of balance, resistance, and flexibility exercises [1, 33, 34]. Two RCT studies focused on investigating the impact of yoga, stretching, movement control exercises, and stability exercises on bone density in osteoporotic patients, with these exercises showing a significant improvement in this variable [35, 36]. Two other RCT studies examined the effects of respiratory, strengthening, balance, range of motion, aerobic, and aquatic exercises compared to land exercises on QoL [13, 37]. A study indicated a noteworthy enhancement in the QoL [37]. Meanwhile, a different study noted a considerable enhancement within every intervention group, including water versus land exercises and the control group; no notable disparity was observed between groups [13].
Muscle strength
In individuals with osteoporosis, the main reason for fractures has been reported to be falling due to impaired balance and reduced muscle strength [42], highlighting the importance of possessing strong lower limb muscles and sustaining a stable balance as crucial factors in independence and the capability to participate in everyday tasks [43]. Hence, enhancing muscle strength and balance can be attained through engaging in sports activities, which has been demonstrated in various studies [44]. In this review study, three studies investigated the impacts of different training on muscle strength in osteoporotic patients. These studies reported a notable increase in muscle power following exercise therapy, which was aligned with the results of the existing review study [1, 33, 34]. One study had a medium effect size [1], while two others had small effect sizes [33, 34]. Exercise can improve muscle strength, balance, postural stability, range of motion, muscle endurance and flexibility by enhancing neuromuscular coordination. Consequently, the incidence of falls and their fractures is reduced in these individuals, improving their QoL [45]. Moreover, improvement in muscle strength in these studies may be linked to the duration of performing these exercises. In other words, consistent and regular exercises enhanced muscle strength in the group receiving exercise therapy as opposed to the control group. In contrast, discontinuing exercise led to a decline in the benefits of the workout. Also, the studies involved a combination of exercises and these combined exercises with effective intensity played an optimal role in increasing muscle strength compared to each exercise separately [46].
Bone density
Tiny structures (organizational spatial structure of bone tissue) and bone mass are important determinants of bone mechanical capability against fractures. It is essential to examine these factors when assessing the impact of aging on bone tissue [47]. With aging and the deterioration of tiny structures, bone fragility occurs in both genders with unequal ratios [47]. Therefore, it is necessary to use treatments to preserve the microscopic structures and improve bone density. This review article includes two studies investigating how exercise therapy affects bone density in osteoporotic individuals [35]. One study with a small effect size [35] and another with a moderate effect size reported a significant improvement aligned with the existing research results [36]. Concerning the interpretation of the effect size categorized as “moderate” in the study by Preisinger et al. which demonstrated a moderate effect size of 0.63, it can be said that in this study, the frequency, duration of exercise, and patient’s adherence to the intervention had a positive impact on the variables. In other words, all participants completed the treatment in this study, which could have played a role in increasing the effect size [36]. Therefore, the impact of exercise therapy interventions cannot be determined without considering the patients’ follow-up rate [48, 49, 50].
Physical activity is beneficial for enhancing bone density and decreasing the likelihood of fractures in osteoporotic individuals through its impact on bone characteristics [51]. The mechanical load applied to bone tissue within the lacunar-canalicular network creates a guardian that plays a role in events that occur within cells, such as heightened levels of internal calcium, matrix creation, osteogenesis, and ultimately contributing to improved bone density [48]. It has also been shown that strength training contributes to changes in bone structure by creating and distributing non-uniform mechanical pressures [52]. In this type of exercise, optimizing mechanical load and high levels of strain leads to improved bone density [53].
QoL
Osteoporosis is a complex condition that affects many facets of a person’s activity [54]. Various studies reported a significant relationship between osteoporosis and a decrease in the QoL for the individual [54]. Decreased QoL caused by osteoporosis is primarily linked to discomfort and limitations in physical abilities resulting from fractures as a consequence of the disease, as well as physical and social impairments in women following vertebral fractures [54]. On the other hand, a decline in overall well-being can stem from the fear of potential fractures that may occur in the future for the individual, or the individual may feel the need to modify their way of life to avoid possible fractures that may occur in the future [55]. In this review study, 2 studies investigated how different exercises impact the QoL in osteoporotic individuals [13, 37]. Both studies reported a small effect size and a significant improvement that aligns with the findings of the existing review research [13, 37].
Reports have shown that exercise therapy and home training can effectively enhance the QoL. This improvement in the QoL has resulted in a decrease in the likelihood of experiencing falls by up to 50% [56]. Moreover, the positive impact of exercise and group activities on mental health indicators and psychosomatic symptoms can improve motivation and encourage patients to participate in exercise programs. Therefore, increasing patients’ physical activity enhances their daily activities and improves their QoL [57]. It has been reported that compared to other interventions, exercise therapy has positive and significant effects in osteoporotic postmenopausal women. Fishman et al. showed a positive change in spine and hip T-score index following a 2-year pilot study. On the other hand, this study reported a greater enhancement in pelvic bone density than in the spine [58]. Soomro et al. examined the impact of physical activity on preventing osteoporosis compared with walking in young women. They did not report a difference in T-score between the two groups during 3 months. Therefore, to investigate the effect of exercise therapy, it is necessary to conduct more studies over a more extended period [59].
We only included randomized clinical trials to assess the quality of studies better. Most research demonstrated moderately high methodological standards and low and moderate effect sizes. Therefore, effect size measurement can ascertain the impacts of exercise treatment. Based on the results of the present review, physiotherapists and other healthcare professionals can expect many benefits following exercise treatment in osteoporotic individuals.
There were limitations in the present systematic review. First, we only considered studies published in English. Second, all the studies in the present study had a PEDro score between 6 and 9, of medium to high quality. The PEDro scale score in the range of more than 5 is a medium to high score. Therefore, in this case, there is a risk regarding the effects related to the quality of the studies. Third, the studies selected for this review had different follow-up periods. Therefore, it is suggested that the evaluated studies should be similar regarding the follow-up and research periods to make comparing the results easier. In addition, most studies had a brief duration of follow-up. Therefore, it is preferable to consider studies with an extended follow-up period. It is also recommended that studies with more participants be selected for final evaluation.
Conclusion
This research demonstrated the impacts of balance, strength, stretching, stability, and movement control exercises on bone mass and muscle strength in osteoporotic postmenopausal women. However, more studies are needed in this field.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.RETECH.REC.1399.1001).
Funding
This research was supported by a research project (No.: 63675 /1399), Funded by Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors' contributions
Conceptualization: Narges Jahantigh Akbari, Hoda Niknam, Sedigheh Sadat Naimi, Bijan Danesh Shahreki, Ali Jahantigh Akbari and Yousef Nooshiravani; Methodology: Narges Jahantigh Akbari, Hoda Niknam, Bijan Danesh Shahreki, Ali Jahantigh Akbari, Nahid Tahan, and Sedigheh Sadat Naimi; Research, review, analysis and drafting: Narges Jahantigh Akbari, Hoda Niknam, Sedigheh Sadat Naimi, Bijan Danesh Shahreki and Negar Asad-Sajjadi; Sources: Narges Jahantigh Akbari and Hoda Niknam; Visualization: Narges Jahantigh Akbari and Hoda Niknam; Supervision: Narges Jahantigh Akbari, Hoda Niknam, and Sedigheh Sadat Naimi; Project management: Narges Jahantigh Akbari, Hoda Niknam, and Bijan Danesh Shahreki; Review, editing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate the Student Research Committee and Head of Research and Technology at Shahid Beheshti University of Medical Sciences for their financial support of this study.
References
- Carter ND, Khan KM, McKay HA, Petit MA, Waterman C, Heinonen A, et al. Community-based exercise program reduces risk factors for falls in 65-to 75-year-old women with osteoporosis: Randomized controlled trial. CMAJ: Canadian Medical Association Journal. 2002; 167(9):997-1004. [PMID]
- Bringhurs FR. Bone and mineral metabolism in health and disease. Harrison’s Principles of Internal Medicine. 2008; 2365-77. [Link]
- Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO Study Group. Osteoporosis International. 1994; 4(6):368-81. [DOI:10.1007/BF01622200] [PMID]
- O’Brien M. Exercise and osteoporosis. Irish Journal of Medical Science. 2001; 170(1):58-62. [DOI:10.1007/BF03167724] [PMID]
- Sioka C, Fotopoulos A, Georgiou A, Xourgia X, Papadopoulos A, Kalef-Ezra J. Age at menarche, age at menopause and duration of fertility as risk factors for osteoporosis. Climacteric. 2010; 13(1):63-71. [DOI:10.3109/13697130903075337] [PMID]
- Leslie M, St Pierre RW. Osteoporosis: Implications for risk reduction in the college setting. Journal of American College Health. 1999; 48(2):67-71. [DOI:10.1080/07448489909595676] [PMID]
- Tsao LI. Relieving discomforts: the help-seeking experiences of Chinese perimenopausal women in Taiwan. Journal of Advanced Nursing. 2002; 39(6):580-8. [DOI:10.1046/j.1365-2648.2002.02327.x] [PMID]
- Kelley GA, Kelley KS. Exercise and bone mineral density at the femoral neck in postmenopausal women: A meta-analysis of controlled clinical trials with individual patient data. American Journal of Obstetrics and Gynecology. 2006; 194(3):760-7. [DOI:10.1016/j.ajog.2005.09.006] [PMID]
- Norris R. Medical costs of osteoporosis. Bone. 1992; 13(Suppl 2):S11-6. [DOI:10.1016/8756-3282(92)90190-8] [PMID]
- Kelley GA, Kelley KS, Tran ZV. Resistance training and bone mineral density in women: A meta-analysis of controlled trials. American Journal of Physical Medicine & Rehabilitation. 2001; 80(1):65-77. [DOI:10.1097/00002060-200101000-00017] [PMID]
- Jang SY, Park J, Ryu SY, Choi SW. Low muscle mass is associated with osteoporosis: A nationwide population-based study. Maturitas. 2020; 133:54-9. [DOI:10.1016/j.maturitas.2020.01.003] [PMID]
- Benedetti MG, Furlini G, Zati A, Letizia Mauro G. The effectiveness of physical exercise on bone density in osteoporotic patients. BioMed Research International. 2018; 2018:4840531.[DOI:10.1155/2018/4840531] [PMID]
- Arnold CM, Busch AJ, Schachter CL, Harrison EL, Olszynski WP. A randomized clinical trial of aquatic versus land exercise to improve balance, function, and quality of life in older women with osteoporosis. Physiotherapy Canada. 2008; 60(4):296-306. [DOI:10.3138/physio.60.4.296] [PMID]
- Cranney A. Treatment of postmenopausal osteoporosis. BMJ. 2003; 327(7411):355-6. [DOI:10.1136/bmj.327.7411.355] [PMID]
- Body JJ, Bergmann P, Boonen S, Boutsen Y, Bruyere O, Devogelaer JP, et al. Non-pharmacological management of osteoporosis: A consensus of the Belgian Bone Club. Osteoporosis International. 2011; 22(11):2769-88. [DOI:10.1007/s00198-011-1545-x] [PMID]
- Khorsandi M, Shamsi M, Jahani F. [The survey of practice about prevention of osteoporosis based on health belief model in pregnant women in Arak city (Persian)]. Journal of Rafsanjan University of Medical Sciences. 2013; 12(1):35-46. [Link]
- Reid IR, Billington EO. Drug therapy for osteoporosis in older adults. The Lancet. 2022; 399(10329):1080-92. [DOI:10.1016/S0140-6736(21)02646-5] [PMID]
- Lin Z, Shi G, Liao X, Huang J, Yu M, Liu W, et al. Correlation between sedentary activity, physical activity and bone mineral density and fat in America: National Health and Nutrition Examination Survey, 2011-2018. Scientific Reports. 2023; 13(1):10054. [DOI:10.1038/s41598-023-35742-z] [PMID]
- Reinoso H, McCaffrey RG, Taylor DW. Mitigating fall risk: A community fall reduction program. Geriatric Nursing. 2018; 39(2):199-203. [DOI:10.1016/j.gerinurse.2017.08.014] [PMID]
- Kistler-Fischbacher M, Weeks BK, Beck BR. The effect of exercise intensity on bone in postmenopausal women (part 2): A meta-analysis. Bone. 2021; 143:115697. [DOI:10.1016/j.bone.2020.115697]
- Shojaa M, Von Stengel S, Schoene D, Kohl M, Barone G, Bragonzoni L, et al. Effect of exercise training on bone mineral density in post-menopausal women: A systematic review and meta-analysis of intervention studies. Frontiers in Physiology. 2020; 11:652. [DOI:10.3389/fphys.2020.00652] [PMID]
- Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW. The effect of exercise training programs on bone mass: A meta-analysis of published controlled trials in pre-and postmenopausal women. Osteoporosis International. 1999; 9(1):1-12. [DOI:10.1007/s001980050109] [PMID]
- Hsu WL, Chen CY, Tsauo JY, Yang RS. Balance control in elderly people with osteoporosis. Journal of the Formosan Medical Association. 2014; 113(6):334-9. [DOI:10.1016/j.jfma.2014.02.006] [PMID]
- Iwamoto J, Yeh JK, Aloia JF. Differential effect of treadmill exercise on three cancellous bone sites in the young growing rat. Bone. 1999; 24(3):163-9. [DOI:10.1016/S8756-3282(98)00189-6] [PMID]
- Hourigan SR, Nitz JC, Brauer SG, O'Neill S, Wong J, Richardson CA. Positive effects of exercise on falls and fracture risk in osteopenic women. Osteoporosis International. 2008; 19(7):1077-86. [DOI:10.1007/s00198-007-0541-7] [PMID]
- Hong AR, Kim SW. Effects of resistance exercise on bone health. Endocrinology and Metabolism. 2018; 33(4):435-44. [DOI:10.3803/EnM.2018.33.4.435] [PMID]
- Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. American Journal of Managed Care. 2011; 17(6):S164-9. [PMID]
- Nikpour S, Haji Kazami E, Haghani H. Study of the kind and time of occupational and leisure physical activities among employed women in faculties of Iran University of Medical Sciences. Razi Journal of Medical Sciences. 2005; 12(46):381-92. [Link]
- Gold T, Williams SA, Weiss RJ, Wang Y, Watkins C, Carroll J, et al. Impact of fractures on quality of life in patients with osteoporosis: A US cross-sectional survey. Journal of Drug Assessment. 2019; 8(1):175-83. [DOI:10.1080/21556660.2019.1677674] [PMID]
- Chandran M, Brind'Amour K, Fujiwara S, Ha YC, Tang H, Hwang JS, et al. Prevalence of osteoporosis and incidence of related fractures in developed economies in the Asia Pacific region: A systematic review. Osteoporosis International. 2023; 34(6):1037-53. [DOI:10.1007/s00198-022-06657-8] [PMID]
- Wilhelm M, Roskovensky G, Emery K, Manno C, Valek K, Cook C. Effect of resistance exercises on function in older adults with osteoporosis or osteopenia: A systematic review. Physiotherapy Canada. 2012; 64(4):386-94. [DOI:10.3138/ptc.2011-31BH] [PMID]
- Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Physical Therapy. 2003; 83(8):713-21. [DOI:10.1093/ptj/83.8.713] [PMID]
- Carter ND, Khan KM, Petit MA, Heinonen A, Waterman C, Donaldson MG, et al. Results of a 10 week community based strength and balance training programme to reduce fall risk factors: a randomised controlled trial in 65-75 year old women with osteoporosis. British Journal of Sports Medicine. 2001; 35(5):348-51. [DOI:10.1136/bjsm.35.5.348] [PMID]
- Otero M, Esain I, González-Suarez ÁM, Gil SM. The effectiveness of a basic exercise intervention to improve strength and balance in women with osteoporosis. Clinical Interventions in Aging. 2017; 12:505-13. [DOI:10.2147/CIA.S127233] [PMID]
- Motorwala ZS, Kolke S, Panchal PY, Bedekar NS, Sancheti PK, Shyam A. Effects of Yogasanas on osteoporosis in postmenopausal women. International Journal of Yoga. 2016; 9(1):44-8. [DOI:10.4103/0973-6131.171717] [PMID]
- Preisinger E, Alacamlioglu Y, Pils K, Bosina E, Metka M, Schneider B, et al. Exercise therapy for osteoporosis: Results of a randomised controlled trial. British Journal of Sports Medicine. 1996; 30(3):209-12. [DOI:10.1136/bjsm.30.3.209] [PMID]
- Koevska V, Nikolikj-Dimitrova E, Mitrevska B, Gjeracaroska-Savevska C, Gocevska M, Kalcovska B. Effect of exercises on quality of life in patients with postmenopausal osteoporosis-randomized trial. Open access Macedonian Journal of Medical Sciences. 2019; 7(7):1160-5. [DOI:10.3889/oamjms.2019.271] [PMID]
- Borer KT. Physical activity in the prevention and amelioration of osteoporosis in women: Interaction of mechanical, hormonal and dietary factors. Sports Medicine. 2005; 35(9):779-830.[DOI:10.2165/00007256-200535090-00004] [PMID]
- Bagheri P, Haghdoost AA, Dortaj Rabari E, halimi L, Vafaei Z, Farhang Nya M, et al. [Ultra analysis of prevalence of osteoporosis in iranian women: A systematic review and meta-analysis (Persian)]. Iranian Journal of Endocrinology & Metabolism. 2011; 13(3):315-25. [Link]
- Borgström F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, Svensson O, et al. Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporosis International. 2006; 17(5):637-50. [DOI:10.1007/s00198-005-0015-8] [PMID]
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporosis International. 2005; 16(Suppl 2):S3-7. [DOI:10.1007/s00198-004-1702-6] [PMID]
- Ganança FF, Gazzola JM, Ganança CF, Caovilla HH, Ganança MM, Cruz OL. Elderly falls associated with benign paroxysmal positional vertigo. Brazilian Journal of Otorhinolaryngology. 2010; 76(1):113-20. [DOI:10.1590/S1808-86942010000100019] [PMID]
- Lee DK, Kang MH, Lee TS, Oh JS. Relationships among the Y balance test, Berg Balance Scale, and lower limb strength in middle-aged and older females. Brazilian Journal of Physical Therapy. 2015; 19(3):227-34. [DOI:10.1590/bjpt-rbf.2014.0096] [PMID]
- Erhan B, Ataker Y. Rehabilitation of patients with osteoporotic fractures. Journal of Clinical Densitometry. 2020; 23(4):534-8. [DOI:10.1016/j.jocd.2020.06.006] [PMID]
- Alonso Pérez JL, Martín Pérez S, Battaglino A, Villafañe JH, Alonso-Sal A, Sánchez Romero EA. An up-date of the muscle strengthening exercise effectiveness in postmenopausal women with osteoporosis: A qualitative systematic review. Journal of Clinical Medicine. 2021; 10(11):2229. [DOI:10.3390/jcm10112229] [PMID]
- Kemmler W, Lauber D, Weineck J, Hensen J, Kalender W, Engelke K. Benefits of 2 years of intense exercise on bone density, physical fitness, and blood lipids in early postmenopausal osteopenic women: Results of the Erlangen Fitness Osteoporosis Prevention Study (EFOPS). Archives of Internal Medicine. 2004; 164(10):1084-91. [DOI:10.1001/archinte.164.10.1084] [PMID]
- Xiang Y, Yingling VR, Malique R, Li CY, Schaffler MB, Raphan T. Comparative assessment of bone mass and structure using texture-based and histomorphometric analyses. Bone. 2007; 40(2):544-52. [DOI:10.1016/j.bone.2006.08.015] [PMID]
- Rampello A, Franceschini M, Piepoli M, Antenucci R, Lenti G, Olivieri D, et al. Effect of aerobic training on walking capacity and maximal exercise tolerance in patients with multiple sclerosis: a randomized crossover controlled study. Physical Therapy. 2007; 87(5):545-55. [DOI:10.2522/ptj.20060085] [PMID]
- Vore ME, Elgelid S, Bolger S, Parsons C, Quashnoc R, Raymor J. Impact of a 10-week individualized exercise program on physical function and fatigue of people with multiple sclerosis: A pilot study. International Journal of MS Care. 2011; 13(3):121-6. [DOI:10.7224/1537-2073-13.3.121] [PMID]
- Sluijs EM, Kok GJ, Van der Zee J. Correlates of exercise compliance in physical therapy. Physical Therapy. 1993; 73(11):771-82. [DOI:10.1093/ptj/73.11.771] [PMID]
- Hoffmann I, Kohl M, von Stengel S, Jakob F, Kerschan-Schindl K, Lange U, et al. Exercise and the prevention of major osteoporotic fractures in adults: a systematic review and meta-analysis with special emphasis on intensity progression and study duration. Osteoporosis International. 2023; 34(1):15-28. [DOI:10.1007/s00198-022-06592-8] [PMID]
- Sinaki M, Pfeifer M, Preisinger E, Itoi E, Rizzoli R, Boonen S, et al. The role of exercise in the treatment of osteoporosis. Current Osteoporosis Reports. 2010; 8(3):138-44. [DOI:10.1007/s11914-010-0019-y] [PMID]
- Schmitt NM, Schmitt J, Dören M. The role of physical activity in the prevention of osteoporosis in postmenopausal women-an update. Maturitas. 2009; 63(1):34-8. [DOI:10.1016/j.maturitas.2009.03.002] [PMID]
- Lips P, Cooper C, Agnusdei D, Caulin F, Egger P, Johnell O, et al. Quality of life in patients with vertebral fractures: Validation of the quality of life questionnaire of the European Foundation for Osteoporosis (QUALEFFO). Osteoporosis International. 1999; 10:150-60. [DOI:10.1007/s001980050210] [PMID]
- Martin AR, Sornay-Rendu E, Chandler JM, Duboeuf F, Girman CJ, Delmas PD. The impact of osteoporosis on quality-of-life: The OFELY cohort. Bone. 2002; 31(1):32-6. [DOI:10.1016/S8756-3282(02)00787-1] [PMID]
- Madureira MM, Bonfá E, Takayama L, Pereira RM. A 12-month randomized controlled trial of balance training in elderly women with osteoporosis: Improvement of quality of life. Maturitas. 2010; 66(2):206-11. [DOI:10.1016/j.maturitas.2010.03.009] [PMID]
- Li WC, Chen YC, Yang RS, Tsauo JY. Effects of exercise programmes on quality of life in osteoporotic and osteopenic postmenopausal women: A systematic review and meta-analysis. Clinical Rehabilitation. 2009; 23(10):888-96. [DOI:10.1177/0269215509339002] [PMID]
- Fishman LM. Yoga for osteoporosis: A pilot study. Topics in Geriatric Rehabilitation. 2009; 25(3):244-50. [DOI:10.1097/TGR.0b013e3181b02dd6]
- Soomro RR, Ahmed SI, Khan M, Ali SS. Comparing the effects of Osteoporosis Prevention Exercise Protocol (OPEP) versus walking in the prevention of osteoporosis in younger females. Pakistan Journal of Medical Sciences. 2015; 31(2):336-40. [DOI:10.12669/pjms.312.5990] [PMID]
Type of Study: Systematic Review |
Subject:
Physical Therapy
Received: 7/07/2023 | Accepted: 13/04/2024 | Published: 1/10/2024
Received: 7/07/2023 | Accepted: 13/04/2024 | Published: 1/10/2024
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |