Volume 24, Issue 3 (Autumn 2023)
jrehab 2023, 24(3): 418-435 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hadizadeh M, Rahimi A, Javaherian M, Velayati M, Naderi F, Dommerholt J. The Effects of Intramuscular Electrical Stimulation on Clinical and Sonographic Parameters in the People With Trigger Points: A Case Series Study. jrehab 2023; 24 (3) :418-435
URL: http://rehabilitationj.uswr.ac.ir/article-1-3202-en.html
URL: http://rehabilitationj.uswr.ac.ir/article-1-3202-en.html
Monavar Hadizadeh1 

 , Abbas Rahimi *2
, Abbas Rahimi *2 

 , Mohammad Javaherian3
, Mohammad Javaherian3 

 , Meysam Velayati4
, Meysam Velayati4 

 , Farokh Naderi5
, Farokh Naderi5 

 , Jan Dommerholt6
, Jan Dommerholt6 




 , Abbas Rahimi *2
, Abbas Rahimi *2 

 , Mohammad Javaherian3
, Mohammad Javaherian3 

 , Meysam Velayati4
, Meysam Velayati4 

 , Farokh Naderi5
, Farokh Naderi5 

 , Jan Dommerholt6
, Jan Dommerholt6 


1- Department of Physiotherapy, School of Rehabilitation, Shahid Beheshti University of Medical Sciences, Tehran, Iran., Hadizade.mahsa1992@yahoo.com
2- Department of Physiotherapy, School of Rehabilitation, Shahid Beheshti University of Medical Sciences, Tehran, Iran. ,a_rahimi@sbmu.ac.ir
3- Department of Physiotherapy, School of Rehabilitation, Tehran University of Medical Sciences, Tehran, Iran., javaherian_m@razi.tums.ac.ir
4- Department of Radiology, Akhtar Orthopedic Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran., Drmeysam.v@gmail.com
5- Department of Radiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran., Dr.naderi@yahoo.com
6- Bethesda Physiocare, Bethesda, United States., jan@myopain4u.com
2- Department of Physiotherapy, School of Rehabilitation, Shahid Beheshti University of Medical Sciences, Tehran, Iran. ,
3- Department of Physiotherapy, School of Rehabilitation, Tehran University of Medical Sciences, Tehran, Iran., javaherian_m@razi.tums.ac.ir
4- Department of Radiology, Akhtar Orthopedic Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran., Drmeysam.v@gmail.com
5- Department of Radiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran., Dr.naderi@yahoo.com
6- Bethesda Physiocare, Bethesda, United States., jan@myopain4u.com
Keywords: Trigger point, Myofascial pain syndrome, Intramuscular electrical stimulation, Ultrasound imaging
Full-Text [PDF 2752 kb]
(1122 Downloads)
| Abstract (HTML) (7414 Views)
Full-Text: (1541 Views)
Introduction
Musculoskeletal pain is one of the most frequent reasons for admitting clinics. This problem is among the most important concerns of human societies [1, 2]. Myofascial pain syndrome (MPS) is a common musculoskeletal pain affecting up to 85% of the population [3-5]. One of the main features of MPS is the common presence of trigger points (TrPs) [3, 6, 7]. These points are usually seen with other diseaese and worsen the symptoms of those complications. It is suggested that after detecting TrPs, they should be treated before or at least at the same time as the accompanying disease [8-11].
Different interventions are employed to reduce and manage the symptoms of MPS. These interventions include manual therapy, electrotherapy, acupuncture, exercise therapy, and drug injections. While physical therapy and manual therapy interventions are generally acceptable, further research should investigate the effects of placebo, the appropriate dosage, and the durability of the therapeutic effects of these interventions [12-17].
Intramuscular electrical stimulation (IMES) is a relatively new treatment method for musculoskeletal disorders. A few studies have evaluated and reported the promising results and effectiveness of this intervention for muscle pain [18-23]. So far, clinical and subjective instruments have been the only methods used to examine changes resulting from this intervention. In other words, no studies has ever used objective methods to determine the exact location of TrP and investigate this intervention’s therapeutic effects.
This complication is barely diagnosed correctly, and therapists alleviate the symptoms of this complication using drug interventions [24]. Until now, no universally accepted objective diagnostic criteria existed for this complication. Usually, TrPs are manually detected [25]. Several studies showed poor reproducibility, and some showed better reproducibility for manual assessment to detect and locate TrPs [26-28]. Based on research, reproducibility depends on the examiner's experience, muscle depth, and active or latent TrP. Manual detection only differentiates affected muscle from healthy one and does not determine the exact location of TrP [29-39]. Some studies have investigated the morphological characteristics and hemodynamics of TrPs with the objective ultrasound tool and shown that this method can improve the accurate diagnosis and evaluation of TrPs [40-42].
Limited studies show the promising effects of IMES. In these studies, only clinical and subjective tools have been used. This clinical study investigated the therapeutic effects of IMES intervention on clinical symptoms and ultrasound imaging parameters related to TrPs in patients with TrPs of the upper trapezius muscle.
Materials and Methods
This investigation is a case series study. People with TrP symptoms in the upper trapezius muscle were invited to the research site. The inclusion criteria included women aged 20-35 years with TrP in the upper trapezius muscle [43], confirmation of TrP as the hypoechoic area in B-mode ultrasound image [44], and pain greater than 3 on the visual analog scale (VAS) associated with TrP of the upper trapezius muscle (moderate pain) [45]. The exclusion criteria included people with muscle disease, fibromyalgia, malignancy, neurological disorders, neck or shoulder surgery, severe fear and poor response to acupuncture [46], use of anticoagulants [46], vascular disease [46], and migraine. Eligible individuals were invited to an imaging center. The existence of a trigger point was evaluated manually and clinically [43]. Then, an experienced radiologist performed the ultrasound. If the presence of TrP was confirmed as a hypoechoic area, the patients were included in the study.
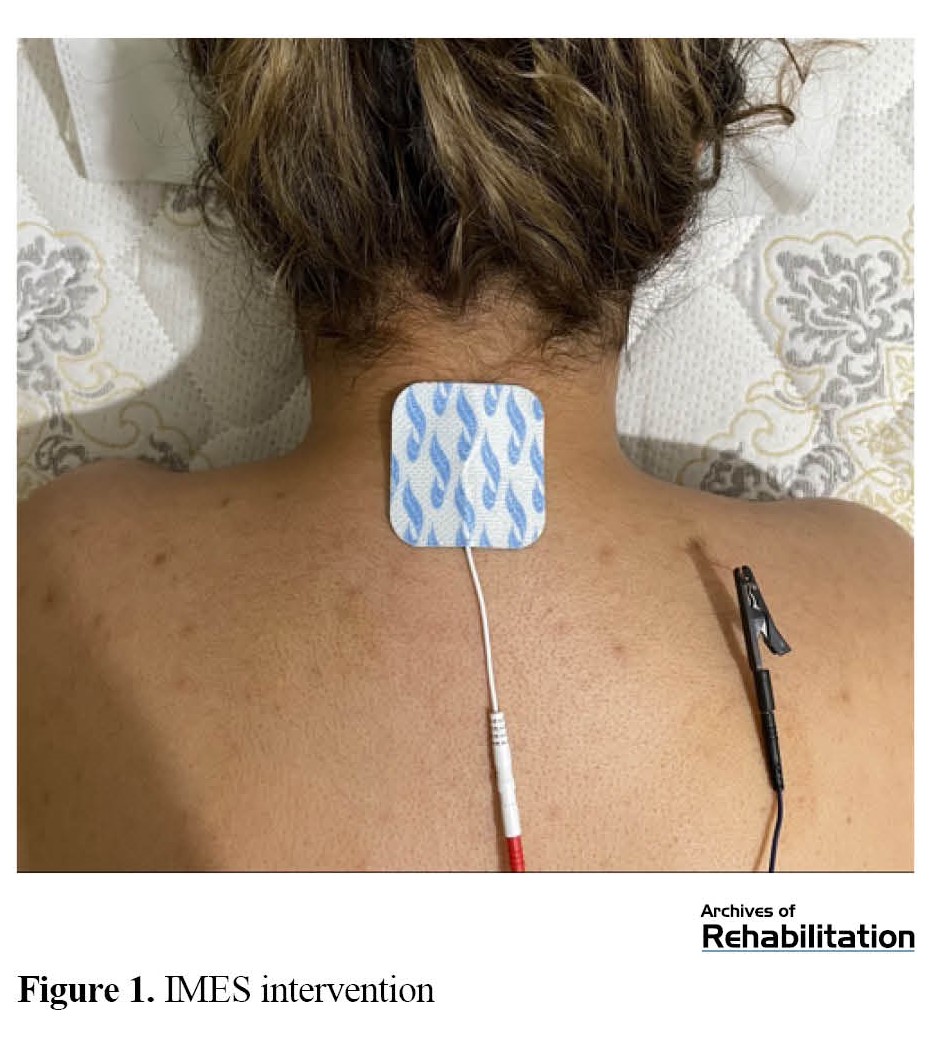
IMES intervention was applied in three sessions during one week. Participants were placed in a prone position, lying on their stomachs. Then, the researcher inserted the dry needle toward the TrP [47]. The cathode was connected to the needle using a pincer electrode, and the anode was placed on the spinous processes of the C7 vertebra using an adhesive electrode. An electric burst current with a frequency of 2 Hz and a pulse width of 200 ms was applied for 10 min [48] (Figure 1). The intensity of the current was increased until a painless contraction. The device used in this study was ES-160 by ITO (Japan).
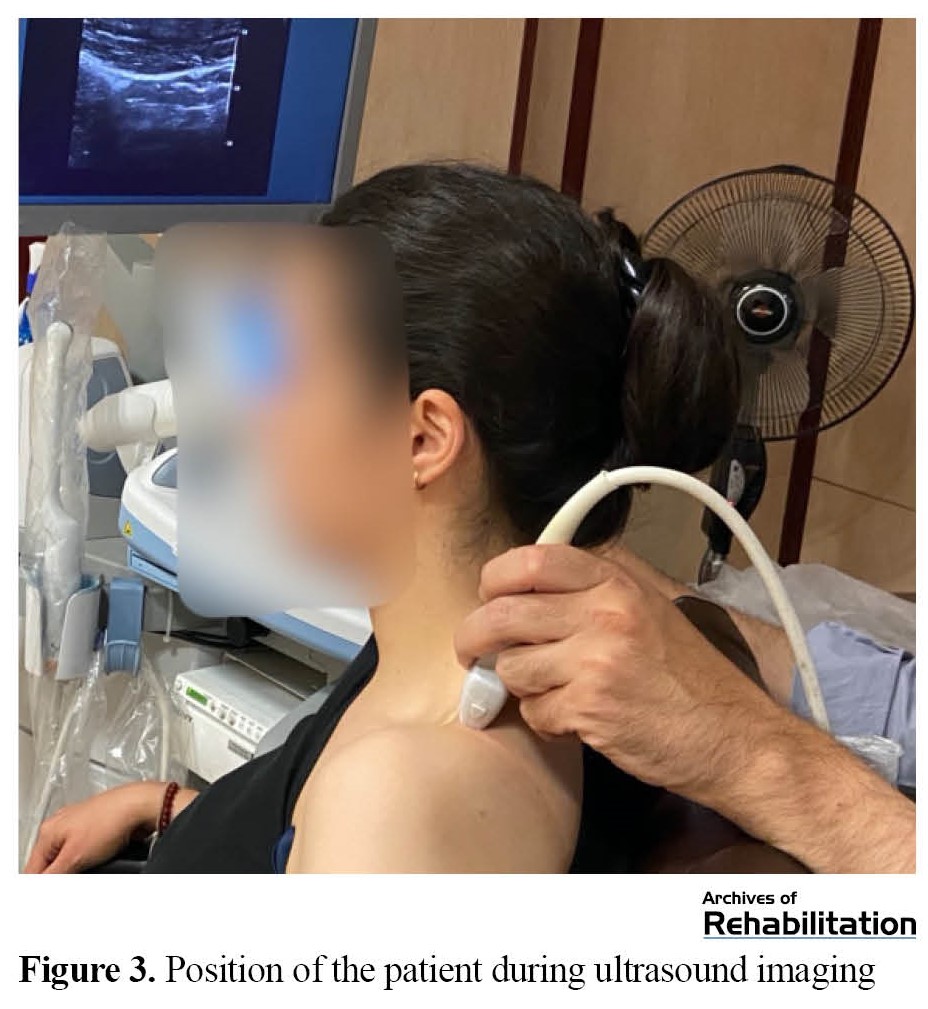
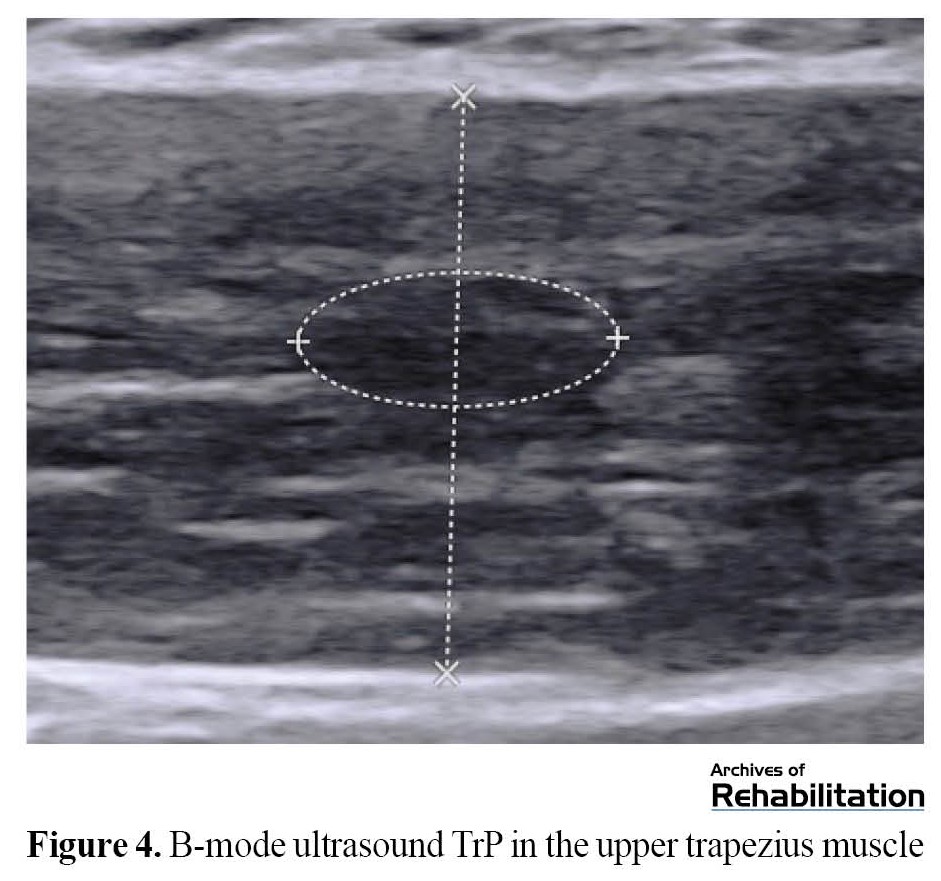
Pain intensity, active rotation of the neck (to the affected side), longitudinal diameter and TrP area, as well as the thickness of the upper trapezius muscle, were measured before the beginning of the sessions and immediately after the last treatment session (the third session) (Figure 2). Pain intensity was measured by VAS. Excellent reproducibility of this tool has been reported to measure acute pain [49-51]. A goniometer was used to measure the movement range of neck rotation [52]. The excellent repeatability of this tool has been reported to measure an active range of motion [53]. Ultrasound images were recorded using a linear probe with a frequency of 4 to 15 MHz (Aixplorer SuperSonic Imagine, France, 2017). The participants sat in a relaxed position (Figure 3). The probe was placed perpendicular to the upper trapezius muscle, parallel to the muscle fibers. Then, the B-mode image was recorded. Longitudinal diameter and area were measured on the frozen image (Figure 4). The vertical distance between two hyperechoic membranes was measured as thickness (Figure 4). Ultrasound is a reproducible technique to measure TrP characteristics [42, 44]. All variables were evaluated three times at each evaluation time, and their average was recorded.
Stata software, version 13 was used to analyze data. Continuous data were reported as Mean±SD. The normality of the data for all variables was examined using the Shapiro-Wilk test. Using the paired t-test, a pre and post comparison was performed for all dependent variables. To report effect estimates, we used mean difference with a 95% CI and the standardized mean difference with a 95% CI using Cohens d effect size. The statistical significance level was determined to be <0.05. Values of 0.20-0.49, 0.50-0.79, 1.19-0.80, and >1.20 are considered small, medium, large, and very large effect sizes, respectively.
Results
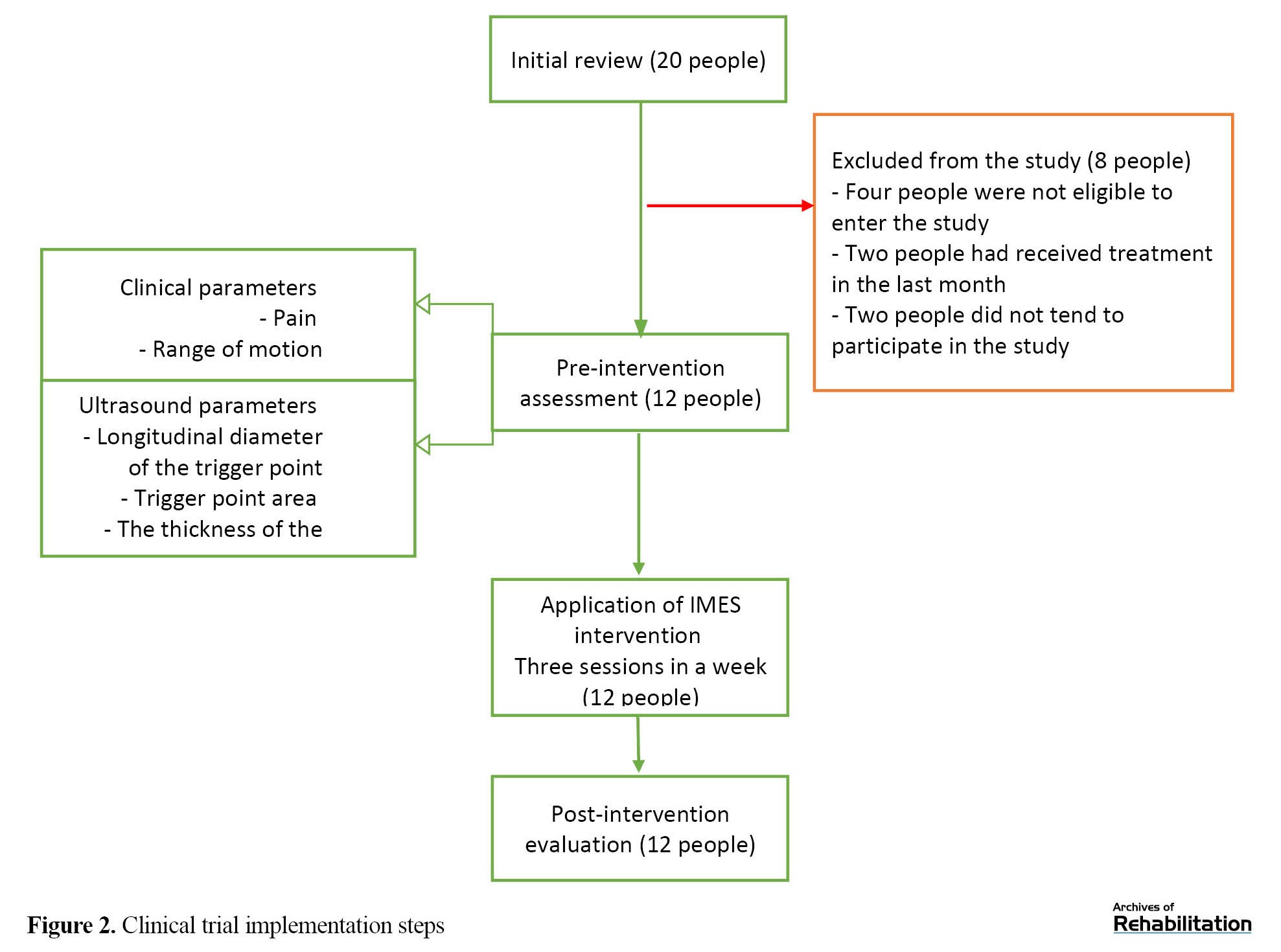
Twenty participants voluntarily participated in this research project for preliminary investigations. Eight participants did not enter the research project due to the exclusion criteria, receiving other treatments one month before the start of the study, or refusing to receive the therapeutic intervention. Finally, 12 female participants with TrPs in the upper trapezius muscles were included in the study (Figure 2). The Mean±SD values of age and duration of symptoms in the participants were 27.25±5.41 years and 12.00±5.72 months, respectively. Table 1 presents the results and changes related to the measured clinical parameters, including the pain and neck rotation range of motion to the same side.
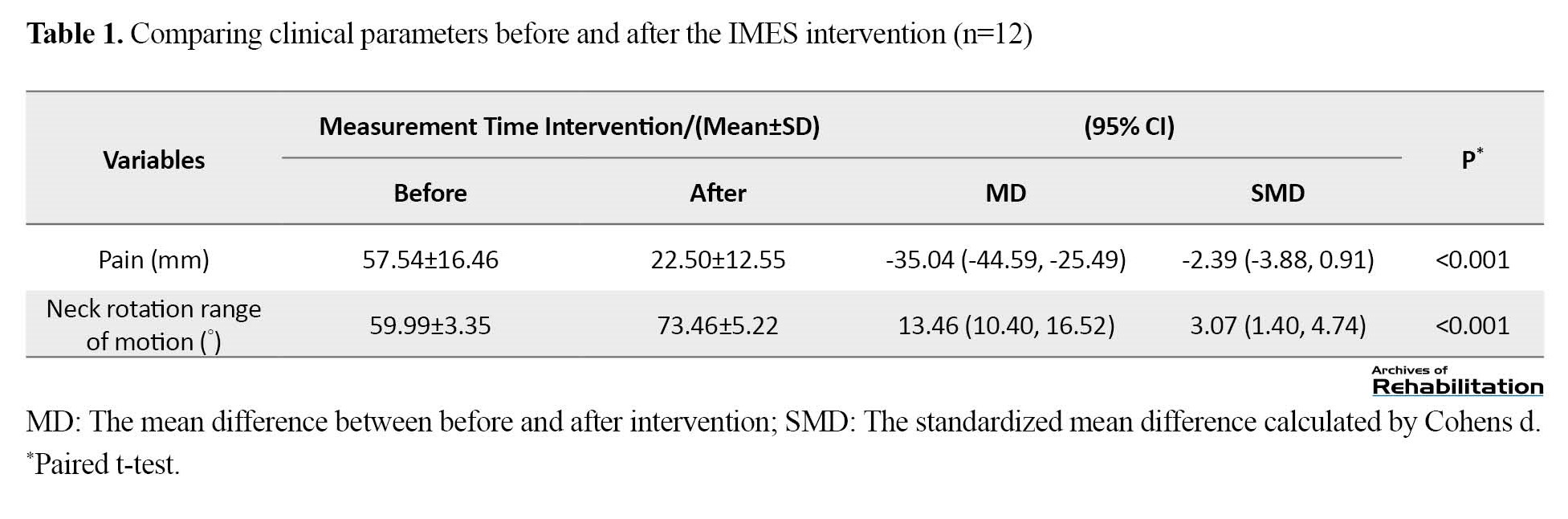
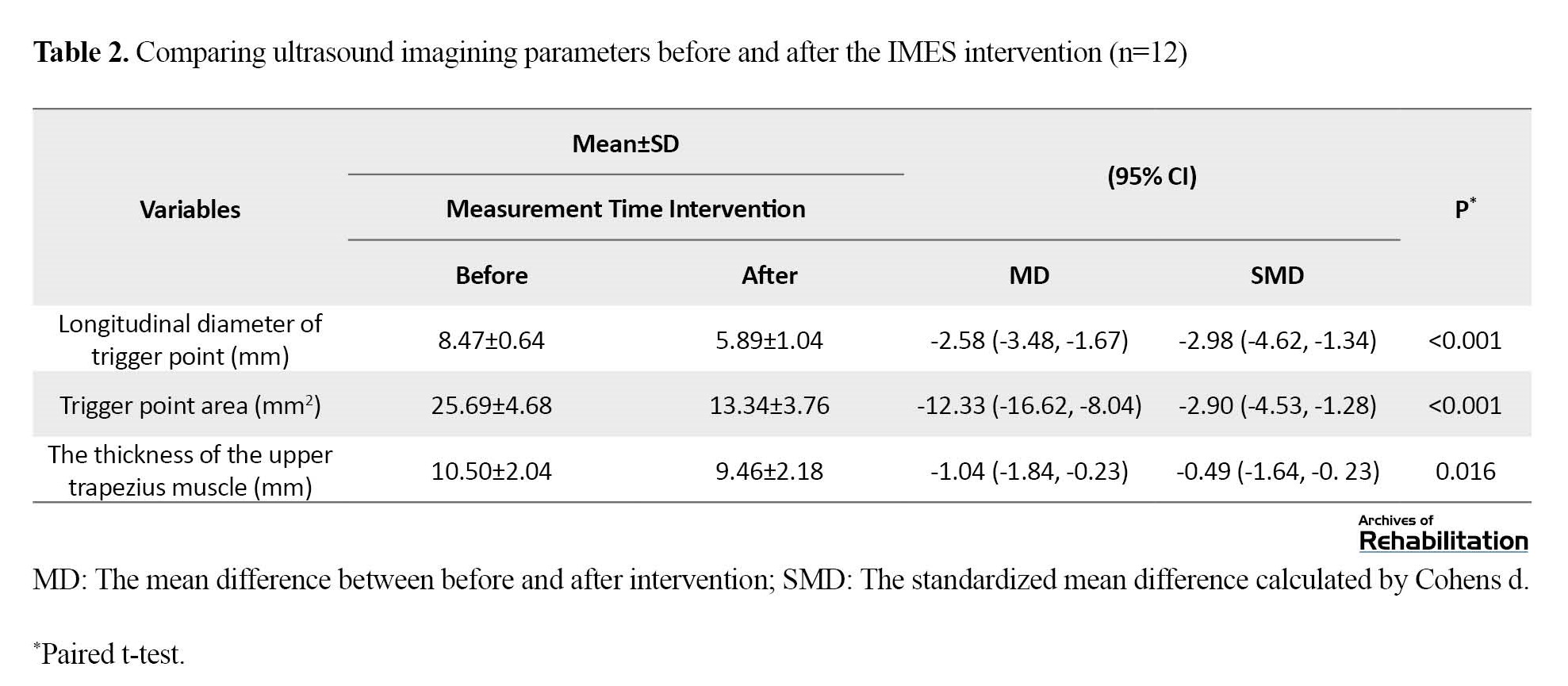
Table 2 presents the results and changes related to the ultrasound imaging parameters, including the longitudinal diameter and TrP area, as well as the thickness of the upper trapezius muscle.
The results of the Shapiro-Wilks test showed that the distribution of all clinical variables and the associated ultrasound imaging was normal (P>0.05). All participants (12 people) completed all treatment sessions (three sessions during one week) and measurements (Figure 2).
According to the results reported in Table 1, the pain intensity changes of the participants decreased after three sessions of IMES intervention, and these changes were significant with a very large effect size (P=0.0000). Also, the movement range of neck rotation to the affected side increased after IMES intervention sessions, and these changes were also significant with a very large effect size (P=0.000).
According to Table 2, the longitudinal diameter and area of the TrP at the time of measurement after three sessions of IMES intervention, have significantly decreased with very large effect size (P=0.0000). Also, the upper trapezius muscle thickness significantly decreased after three intervention sessions, with a small effect size (P=0.016).
Discussion
To the best of research teams knowledge, this study is the first that examines the effects of IMES intervention on ultrasound imaging parameters as an objective tool along with clinical variables in patients with TrPs in their upper trapezius muscles. The results showed that the neck rotation range motion and pain improved after the therapeutic intervention compared to pre-intervention. IMES reduced pain and increased neck rotation range of motion, with very large effect sizes. Regarding the ultrasound evaluation, the longitudinal diameter and TrP area decreased significantly with a very large effect size after three sessions of IMES intervention. However, the thickness of the upper trapezius muscle is reduced with a small effect size. This study provides promising objective effects for IMES intervention in patients with TrP of the upper trapezius muscle.
According to the integrated TrP hypothesis, an abnormal release of acetylcholine exists in the TrP area, which increases the tension of muscle fibers and causes capillary compression in the region; thus, metabolic demands increase and lead to the occurrence of TrP. These events lead to ischemia in the TrP area. This hypoxia causes cell destruction and the release of sensitizing substances, such as substance P, and stimulation of pain receptors and finally leads to pain and sensitivity to touch in the area. The integrated hypothesis offers positive feedback for TrP persistence. Studies have reported that this feedback must be broken to treat TrP [54].
An electrical burst current leading to contraction increases muscle blood flow and decreases static blood flow [48, 55-57]. Increased oxygenation reduces the overlap of muscle fibers (actin and myosin), leading to decreased hypoxia and, thus, the concentration of chemicals in the area. As a result, the stimulation of pain receptors by chemicals is reduced. Since the current used in the present study leads to contraction in the target muscle, probably one of the effective mechanisms of this intervention is due to increasing blood flow and thus reducing hypoxia. On the other hand, peripheral receptors of opioids also seem to be crucial in reducing pain by electrical stimulation with low frequencies [58]. Considering that the frequency used in this study was 2 Hz, this mechanism is probably another reason for improving pain and symptoms caused by this intervention.
Four studies have investigated the effects of this intervention on TrP symptoms [21-24]. In 2008, Lee et al. examined the impact of this intervention on the TrP of the upper trapezius muscle and the levator scapula and reported promising effects. Although objective tools were not used in this study, the results were consistent with the current research [21]. In another study, researchers investigated the effects of this intervention on thoracic pain and reported positive outcomes for pain relief [22]. In 2015, Sumen et al. investigated the impacts of IMES compared to low-level laser on TrP of the upper trapezius muscle. Researchers reported positive effects of this intervention with exercise therapy. Although the current used in this study has not led to muscle contraction, the results of this study are also consistent with our research [23]. In 2017, a study examined the effectiveness of this intervention compared to the placebo group and reported improvement in pain and range of motion [24].
Overall, previous studies have investigated the effect of IMES intervention on TrP only with clinical and subjective parameters, such as pain and range of motion. The results of these studies are consistent with the present study, and they have reported promising effects of this intervention on the clinical symptoms of people with TrPs. None of the previous studies have investigated the impact of this intervention using objective parameters. In the current study, ultrasound imaging parameters are also examined in addition to reviewing clinical parameters. The results show promising and positive effects on the investigated parameters.
Studies have shown that muscle ultrasound is a reliable tool to examine TrP characteristics, such as size, stiffness, and blood flow, and can also help correctly diagnose the presence of TrP [42, 44, 59]. Some studies have investigated the effects of dry needling on TrP using ultrasound. These studies investigated size, stiffness, and blood flow [60, 61]. To the best of the research team’s knowledge, this study is the first to examine the effects of IMES intervention on TrP using ultrasound imaging as an objective tool. The results showed promising effects for this intervention on ultrasound parameters in people with TrP of the upper trapezius muscle.
This study had several limitations. The small sample size of this study prevents a definitive conclusion. No other control or comparison group exists to compare its effectiveness with the IMES intervention. Only the immediate effects of this intervention on people have been examined in this study, and the long-term results of this type of intervention require separate studies.
Conclusion
This study shows that three sessions of IMES intervention in people with TrP of the upper trapezius muscle may have promising effects on reducing pain, increasing range of motion, and improving ultrasound parameters, including TrP size and muscle thickness. The impact of this intervention on the TrP of the upper trapezius muscle has not been determined definitively yet. Further research with a larger sample size and control group is needed to evaluate the long-term effectiveness of this intervention compared to other interventions on patients with TrP of the upper trapezius muscle.
Ethical Considerations
Compliance with ethical guidelines
Ethical approval was obtained from Shahid Beheshti University of Medical Sciences (Code: IR.SBMU.RETECH.REC.1399.480). All participants were informed about the study objectives and methods and signed a written consent form. They were assured of the confidentiality of their information and were free to leave at any time.
Funding
This article was extracted from the PhD thesis of Monavar Hadizadeh, approved by Department of Physiotherapy, School of Rehabilitation, Shahid Beheshti University of Medical Sciences.
Authors' contributions
Conceptualization: Monavar Hadizadeh, Abbas Rahimi, Meisam Velayati, Farrokh Naderi and John Damerholt; Methodology: Manovar Hadizadeh, Abbas Rahimi, Farrokh Naderi and John Damerholt; Research and review, analysis, drafting: Manovar Hadizadeh and Mohammad Javaherian; Sources: Manavar Hadizadeh; Visualization: Monavar Hadizadeh and Meysam Velayati; Supervision: Monavar Hadizadeh, Abbas Rahimi and Farrokh Naderi; Project management: Monavar Hadizadeh and Abbas Rahimi; Editing and finalization: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The present article is part of a PhD thesis and was partially supported by the School of Rehabilitation, Shahid Beheshti University of Medical Sciences.
References
Musculoskeletal pain is one of the most frequent reasons for admitting clinics. This problem is among the most important concerns of human societies [1, 2]. Myofascial pain syndrome (MPS) is a common musculoskeletal pain affecting up to 85% of the population [3-5]. One of the main features of MPS is the common presence of trigger points (TrPs) [3, 6, 7]. These points are usually seen with other diseaese and worsen the symptoms of those complications. It is suggested that after detecting TrPs, they should be treated before or at least at the same time as the accompanying disease [8-11].
Different interventions are employed to reduce and manage the symptoms of MPS. These interventions include manual therapy, electrotherapy, acupuncture, exercise therapy, and drug injections. While physical therapy and manual therapy interventions are generally acceptable, further research should investigate the effects of placebo, the appropriate dosage, and the durability of the therapeutic effects of these interventions [12-17].
Intramuscular electrical stimulation (IMES) is a relatively new treatment method for musculoskeletal disorders. A few studies have evaluated and reported the promising results and effectiveness of this intervention for muscle pain [18-23]. So far, clinical and subjective instruments have been the only methods used to examine changes resulting from this intervention. In other words, no studies has ever used objective methods to determine the exact location of TrP and investigate this intervention’s therapeutic effects.
This complication is barely diagnosed correctly, and therapists alleviate the symptoms of this complication using drug interventions [24]. Until now, no universally accepted objective diagnostic criteria existed for this complication. Usually, TrPs are manually detected [25]. Several studies showed poor reproducibility, and some showed better reproducibility for manual assessment to detect and locate TrPs [26-28]. Based on research, reproducibility depends on the examiner's experience, muscle depth, and active or latent TrP. Manual detection only differentiates affected muscle from healthy one and does not determine the exact location of TrP [29-39]. Some studies have investigated the morphological characteristics and hemodynamics of TrPs with the objective ultrasound tool and shown that this method can improve the accurate diagnosis and evaluation of TrPs [40-42].
Limited studies show the promising effects of IMES. In these studies, only clinical and subjective tools have been used. This clinical study investigated the therapeutic effects of IMES intervention on clinical symptoms and ultrasound imaging parameters related to TrPs in patients with TrPs of the upper trapezius muscle.
Materials and Methods
This investigation is a case series study. People with TrP symptoms in the upper trapezius muscle were invited to the research site. The inclusion criteria included women aged 20-35 years with TrP in the upper trapezius muscle [43], confirmation of TrP as the hypoechoic area in B-mode ultrasound image [44], and pain greater than 3 on the visual analog scale (VAS) associated with TrP of the upper trapezius muscle (moderate pain) [45]. The exclusion criteria included people with muscle disease, fibromyalgia, malignancy, neurological disorders, neck or shoulder surgery, severe fear and poor response to acupuncture [46], use of anticoagulants [46], vascular disease [46], and migraine. Eligible individuals were invited to an imaging center. The existence of a trigger point was evaluated manually and clinically [43]. Then, an experienced radiologist performed the ultrasound. If the presence of TrP was confirmed as a hypoechoic area, the patients were included in the study.
IMES intervention was applied in three sessions during one week. Participants were placed in a prone position, lying on their stomachs. Then, the researcher inserted the dry needle toward the TrP [47]. The cathode was connected to the needle using a pincer electrode, and the anode was placed on the spinous processes of the C7 vertebra using an adhesive electrode. An electric burst current with a frequency of 2 Hz and a pulse width of 200 ms was applied for 10 min [48] (Figure 1). The intensity of the current was increased until a painless contraction. The device used in this study was ES-160 by ITO (Japan).
Pain intensity, active rotation of the neck (to the affected side), longitudinal diameter and TrP area, as well as the thickness of the upper trapezius muscle, were measured before the beginning of the sessions and immediately after the last treatment session (the third session) (Figure 2). Pain intensity was measured by VAS. Excellent reproducibility of this tool has been reported to measure acute pain [49-51]. A goniometer was used to measure the movement range of neck rotation [52]. The excellent repeatability of this tool has been reported to measure an active range of motion [53]. Ultrasound images were recorded using a linear probe with a frequency of 4 to 15 MHz (Aixplorer SuperSonic Imagine, France, 2017). The participants sat in a relaxed position (Figure 3). The probe was placed perpendicular to the upper trapezius muscle, parallel to the muscle fibers. Then, the B-mode image was recorded. Longitudinal diameter and area were measured on the frozen image (Figure 4). The vertical distance between two hyperechoic membranes was measured as thickness (Figure 4). Ultrasound is a reproducible technique to measure TrP characteristics [42, 44]. All variables were evaluated three times at each evaluation time, and their average was recorded.
Stata software, version 13 was used to analyze data. Continuous data were reported as Mean±SD. The normality of the data for all variables was examined using the Shapiro-Wilk test. Using the paired t-test, a pre and post comparison was performed for all dependent variables. To report effect estimates, we used mean difference with a 95% CI and the standardized mean difference with a 95% CI using Cohens d effect size. The statistical significance level was determined to be <0.05. Values of 0.20-0.49, 0.50-0.79, 1.19-0.80, and >1.20 are considered small, medium, large, and very large effect sizes, respectively.
Results
Twenty participants voluntarily participated in this research project for preliminary investigations. Eight participants did not enter the research project due to the exclusion criteria, receiving other treatments one month before the start of the study, or refusing to receive the therapeutic intervention. Finally, 12 female participants with TrPs in the upper trapezius muscles were included in the study (Figure 2). The Mean±SD values of age and duration of symptoms in the participants were 27.25±5.41 years and 12.00±5.72 months, respectively. Table 1 presents the results and changes related to the measured clinical parameters, including the pain and neck rotation range of motion to the same side.

Table 2 presents the results and changes related to the ultrasound imaging parameters, including the longitudinal diameter and TrP area, as well as the thickness of the upper trapezius muscle.

The results of the Shapiro-Wilks test showed that the distribution of all clinical variables and the associated ultrasound imaging was normal (P>0.05). All participants (12 people) completed all treatment sessions (three sessions during one week) and measurements (Figure 2).
According to the results reported in Table 1, the pain intensity changes of the participants decreased after three sessions of IMES intervention, and these changes were significant with a very large effect size (P=0.0000). Also, the movement range of neck rotation to the affected side increased after IMES intervention sessions, and these changes were also significant with a very large effect size (P=0.000).
According to Table 2, the longitudinal diameter and area of the TrP at the time of measurement after three sessions of IMES intervention, have significantly decreased with very large effect size (P=0.0000). Also, the upper trapezius muscle thickness significantly decreased after three intervention sessions, with a small effect size (P=0.016).
Discussion
To the best of research teams knowledge, this study is the first that examines the effects of IMES intervention on ultrasound imaging parameters as an objective tool along with clinical variables in patients with TrPs in their upper trapezius muscles. The results showed that the neck rotation range motion and pain improved after the therapeutic intervention compared to pre-intervention. IMES reduced pain and increased neck rotation range of motion, with very large effect sizes. Regarding the ultrasound evaluation, the longitudinal diameter and TrP area decreased significantly with a very large effect size after three sessions of IMES intervention. However, the thickness of the upper trapezius muscle is reduced with a small effect size. This study provides promising objective effects for IMES intervention in patients with TrP of the upper trapezius muscle.
According to the integrated TrP hypothesis, an abnormal release of acetylcholine exists in the TrP area, which increases the tension of muscle fibers and causes capillary compression in the region; thus, metabolic demands increase and lead to the occurrence of TrP. These events lead to ischemia in the TrP area. This hypoxia causes cell destruction and the release of sensitizing substances, such as substance P, and stimulation of pain receptors and finally leads to pain and sensitivity to touch in the area. The integrated hypothesis offers positive feedback for TrP persistence. Studies have reported that this feedback must be broken to treat TrP [54].
An electrical burst current leading to contraction increases muscle blood flow and decreases static blood flow [48, 55-57]. Increased oxygenation reduces the overlap of muscle fibers (actin and myosin), leading to decreased hypoxia and, thus, the concentration of chemicals in the area. As a result, the stimulation of pain receptors by chemicals is reduced. Since the current used in the present study leads to contraction in the target muscle, probably one of the effective mechanisms of this intervention is due to increasing blood flow and thus reducing hypoxia. On the other hand, peripheral receptors of opioids also seem to be crucial in reducing pain by electrical stimulation with low frequencies [58]. Considering that the frequency used in this study was 2 Hz, this mechanism is probably another reason for improving pain and symptoms caused by this intervention.
Four studies have investigated the effects of this intervention on TrP symptoms [21-24]. In 2008, Lee et al. examined the impact of this intervention on the TrP of the upper trapezius muscle and the levator scapula and reported promising effects. Although objective tools were not used in this study, the results were consistent with the current research [21]. In another study, researchers investigated the effects of this intervention on thoracic pain and reported positive outcomes for pain relief [22]. In 2015, Sumen et al. investigated the impacts of IMES compared to low-level laser on TrP of the upper trapezius muscle. Researchers reported positive effects of this intervention with exercise therapy. Although the current used in this study has not led to muscle contraction, the results of this study are also consistent with our research [23]. In 2017, a study examined the effectiveness of this intervention compared to the placebo group and reported improvement in pain and range of motion [24].
Overall, previous studies have investigated the effect of IMES intervention on TrP only with clinical and subjective parameters, such as pain and range of motion. The results of these studies are consistent with the present study, and they have reported promising effects of this intervention on the clinical symptoms of people with TrPs. None of the previous studies have investigated the impact of this intervention using objective parameters. In the current study, ultrasound imaging parameters are also examined in addition to reviewing clinical parameters. The results show promising and positive effects on the investigated parameters.
Studies have shown that muscle ultrasound is a reliable tool to examine TrP characteristics, such as size, stiffness, and blood flow, and can also help correctly diagnose the presence of TrP [42, 44, 59]. Some studies have investigated the effects of dry needling on TrP using ultrasound. These studies investigated size, stiffness, and blood flow [60, 61]. To the best of the research team’s knowledge, this study is the first to examine the effects of IMES intervention on TrP using ultrasound imaging as an objective tool. The results showed promising effects for this intervention on ultrasound parameters in people with TrP of the upper trapezius muscle.
This study had several limitations. The small sample size of this study prevents a definitive conclusion. No other control or comparison group exists to compare its effectiveness with the IMES intervention. Only the immediate effects of this intervention on people have been examined in this study, and the long-term results of this type of intervention require separate studies.
Conclusion
This study shows that three sessions of IMES intervention in people with TrP of the upper trapezius muscle may have promising effects on reducing pain, increasing range of motion, and improving ultrasound parameters, including TrP size and muscle thickness. The impact of this intervention on the TrP of the upper trapezius muscle has not been determined definitively yet. Further research with a larger sample size and control group is needed to evaluate the long-term effectiveness of this intervention compared to other interventions on patients with TrP of the upper trapezius muscle.
Ethical Considerations
Compliance with ethical guidelines
Ethical approval was obtained from Shahid Beheshti University of Medical Sciences (Code: IR.SBMU.RETECH.REC.1399.480). All participants were informed about the study objectives and methods and signed a written consent form. They were assured of the confidentiality of their information and were free to leave at any time.
Funding
This article was extracted from the PhD thesis of Monavar Hadizadeh, approved by Department of Physiotherapy, School of Rehabilitation, Shahid Beheshti University of Medical Sciences.
Authors' contributions
Conceptualization: Monavar Hadizadeh, Abbas Rahimi, Meisam Velayati, Farrokh Naderi and John Damerholt; Methodology: Manovar Hadizadeh, Abbas Rahimi, Farrokh Naderi and John Damerholt; Research and review, analysis, drafting: Manovar Hadizadeh and Mohammad Javaherian; Sources: Manavar Hadizadeh; Visualization: Monavar Hadizadeh and Meysam Velayati; Supervision: Monavar Hadizadeh, Abbas Rahimi and Farrokh Naderi; Project management: Monavar Hadizadeh and Abbas Rahimi; Editing and finalization: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The present article is part of a PhD thesis and was partially supported by the School of Rehabilitation, Shahid Beheshti University of Medical Sciences.
References
- Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: A prevalence study. Pain. 2001; 89(2-3):127-34. [DOI:10.1016/S0304-3959(00)00355-9] [PMID]
- Magni G, Caldieron C, Rigatti-Luchini S, Merskey H. Chronic musculoskeletal pain and depressive symptoms in the general population. An analysis of the 1st National Health and Nutrition Examination Survey data. Pain. 1990; 43(3):299-307. [DOI:10.1016/0304-3959(90)90027-B] [PMID]
- Mense S, Simons DG, Russell IJ. Muscle pain: Understanding its nature, diagnosis, and treatment. Pennsylvania: Lippincott Williams & Wilkins; 2001. [Link]
- Simons DG. Understanding effective treatments of myofascial trigger points. Journal of Bodywork and Movement Therapies. 2002; 6(2):81-8. [DOI:10.1054/jbmt.2002.0271]
- Zhuang X, Tan S, Huang Q. Understanding of myofascial trigger points. Chinese Medical Journal. 2014; 127(24):4271-7. [PMID]
- Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. Journal of Electromyography and Kinesiology. 2004; 14(1):95-107. [DOI:10.1016/j.jelekin.2003.09.018] [PMID]
- Travell JG, Simons DG. Myofascial pain and dysfunction: The trigger point manual. Pennsylvania: Lippincott Williams & Wilkins; 1992. [Link]
- Mense S, Gerwin RD. Muscle pain: Understanding the mechanisms. Berlin: Springer; 2010. [DOI:10.1007/978-3-540-85021-2]
- Sergienko S, Kalichman L. Myofascial origin of shoulder pain: A literature review. Journal of Bodywork and Movement Therapies. 2015; 19(1):91-101. [DOI:10.1016/j.jbmt.2014.05.004] [PMID]
- Chiarotto A, Clijsen R, Fernandez-de-Las-Penas C, Barbero M. Prevalence of myofascial trigger points in spinal disorders: A systematic review and meta-analysis. Archives of Physical Medicine and Rehabilitation. 2016; 97(2):316-37. [DOI:10.1016/j.apmr.2015.09.021] [PMID]
- Alburquerque-García A, Rodrigues-de-Souza DP, Fernández-de-las-Peñas C, Alburquerque-Sendín F. Association between muscle trigger points, ongoing pain, function, and sleep quality in elderly women with bilateral painful knee osteoarthritis. Journal of Manipulative and Physiological Therapeutics. 2015; 38(4):262-8. [DOI:10.1016/j.jmpt.2014.10.018] [PMID]
- Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, Del-Moral-Ávila R, Menjón-Beltrán S, Arroyo-Morales M. Development of active myofascial trigger points in neck and shoulder musculature is similar after lumpectomy or mastectomy surgery for breast cancer. Journal of Bodywork and Movement Therapies. 2012; 16(2):183-90. [DOI:10.1016/j.jbmt.2011.01.022] [PMID]
- Giamberardino MA, Affaitati G, Fabrizio A, Costantini R. Myofascial pain syndromes and their evaluation. Best Practice & Research Clinical Rheumatology. 2011; 25(2):185-98. [DOI:10.1016/j.berh.2011.01.002] [PMID]
- Vernon H, Schneider M. Chiropractic management of myofascial trigger points and myofascial pain syndrome: A systematic review of the literature. Journal of Manipulative and Physiological Therapeutics. 2009; 32(1):14-24. [DOI:10.1016/j.jmpt.2008.06.012] [PMID]
- Zhou JY, Wang D. An update on botulinum toxin A injections of trigger points for myofascial pain. Current Pain and Headache Reports. 2014; 18(1):386. [DOI:10.1007/s11916-013-0386-z] [PMID]
- Ong J, Claydon LS. The effect of dry needling for myofascial trigger points in the neck and shoulders: A systematic review and meta-analysis. Journal of Bodywork and Movement Therapies. 2014; 18(3):390-8. [DOI:10.1016/j.jbmt.2013.11.009] [PMID]
- Srbely JZ. New trends in the treatment and management of myofascial pain syndrome. Current Pain and Headache Reports. 2010; 14(5):346-52. [DOI:10.1007/s11916-010-0128-4] [PMID]
- Benjaboonyanupap D, Paungmali A, Pirunsan U. Effect of therapeutic sequence of hot pack and ultrasound on physiological response over trigger point of upper trapezius. Asian Journal of Sports Medicine. 2015; 6(3):e23806. [DOI:10.5812/asjsm.23806] [PMID]
- Chu J, Takehara I, Li T, Schwartz I. Electrical twitch obtaining intramuscular stimulation (ETOIMS) for myofascial pain syndrome in a football player. British Journal of Sports Medicine. 2004; 38(5):E25. [DOI:10.1136/bjsm.2003.010306] [PMID]
- Chu J, Yuen KF, Wang BH, Chan RC, Schwartz I, Neuhauser D. Electrical twitch-obtaining intramuscular stimulation in lower back pain: A pilot study. American Journal of Physical Medicine & Rehabilitation. 2004; 83(2):104-11. [DOI:10.1097/01.PHM.0000107485.86594.8B] [PMID]
- Lee SH, Chen CC, Lee CS, Lin TC, Chan RC. Effects of needle electrical intramuscular stimulation on shoulder and cervical myofascial pain syndrome and microcirculation. Journal of The Chinese Medical Association. 2008; 71(4):200-6. [DOI:10.1016/S1726-4901(08)70104-7] [PMID]
- Rock JM, Rainey CE. Treatment of nonspecific thoracic spine pain with trigger point dry needling and intramuscular electrical stimulation: A case series. International Journal of Sports Physical Therapy. 2014; 9(5):699-711. [PMID]
- Sumen A, Sarsan A, Alkan H, Yildiz N, Ardic F. Efficacy of low level laser therapy and intramuscular electrical stimulation on myofascial pain syndrome. Journal of Back and Musculoskeletal Rehabilitation. 2015; 28(1):153-8. [DOI:10.3233/BMR-140503] [PMID]
- Hadizadeh M, Tajali SB, Moghadam BA, Jalaie S, Bazzaz M. Effects of intramuscular electrical stimulation on symptoms following trigger points; A controlled pilot study. Journal of Modern Rehabilitation. 2017; 11(1):31-6. [DOI:10.18869/nirp.jmr.11.1.31]
- Gerber NL, Sikdar S, Hammond J, Shah J. A brief overview and update of myofascial pain syndrome and myofascial trigger points. Journal of The Spinal Research Foundation. 2011; 6(1):55-64. [Link]
- Vulfsons S, Ratmansky M, Kalichman L. Trigger point needling: Techniques and outcome. Current Pain and Headache Reports. 2012; 16(5):407-12. [DOI:10.1007/s11916-012-0279-6] [PMID]
- Rathbone AT, Grosman-Rimon L, Kumbhare DA. Interrater agreement of manual palpation for identification of myofascial trigger points: A systematic review and meta-analysis. The Clinical Journal of Pain. 2017; 33(8):715-29. [DOI:10.1097/AJP.0000000000000459] [PMID]
- Hsieh CY, Hong CZ, Adams AH, Platt KJ, Danielson CD, Hoehler FK, et al. Interexaminer reliability of the palpation of trigger points in the trunk and lower limb muscles. Archives of Physical Medicine and Rehabilitation. 2000; 81(3):258-64. [DOI:10.1016/S0003-9993(00)90068-6] [PMID]
- Lucas N, Macaskill P, Irwig L, Moran R, Bogduk N. Reliability of physical examination for diagnosis of myofascial trigger points: A systematic review of the literature. The Clinical Journal of Pain. 2009; 25(1):80-9. [DOI:10.1097/AJP.0b013e31817e13b6] [PMID]
- Bron C, Franssen J, Wensing M, Oostendorp RA. Interrater reliability of palpation of myofascial trigger points in three shoulder muscles. Journal of Manual & Manipulative Therapy. 2007; 15(4):203-15. [DOI:10.1179/106698107790819477] [PMID]
- Gerber LH, Sikdar S, Armstrong K, Diao G, Heimur J, Kopecky J, et al. A systematic comparison between subjects with no pain and pain associated with active myofascial trigger points. PM&R. 2013; 5(11):931-8. [DOI:10.1016/j.pmrj.2013.06.006] [PMID]
- Myburgh C, Lauridsen HH, Larsen AH, Hartvigsen J. Standardized manual palpation of myofascial trigger points in relation to neck/shoulder pain; the influence of clinical experience on inter-examiner reproducibility. Manual Therapy. 2011; 16(2):136-40. [DOI:10.1016/j.math.2010.08.002] [PMID]
- Mora-Relucio R, Núñez-Nagy S, Gallego-Izquierdo T, Rus A, Plaza-Manzano G, Romero-Franco N, et al. Experienced versus inexperienced interexaminer reliability on location and classification of myofascial trigger point palpation to diagnose lateral epicondylalgia: an observational cross-sectional study. Evidence-Based Complementary and Alternative Medicine. 2016; 2016:6059719. [DOI:10.1155/2016/6059719] [PMID]
- Sanz DR, Lobo CC, López DL, Morales CR, Marín CS, Corbalán IS. Interrater reliability in the clinical evaluation of myofascial trigger points in three ankle muscles. Journal of Manipulative and Physiological Therapeutics. 2016; 39(9):623-34. [DOI:10.1016/j.jmpt.2016.09.002] [PMID]
- Nascimento JDSD, Alburquerque-Sendín F, Vigolvino LP, Oliveira WF, Sousa CO. Inter-and intraexaminer reliability in identifying and classifying myofascial trigger points in shoulder muscles. Archives of Physical Medicine and Rehabilitation. 2018; 99(1):49-56. [DOI:10.1016/j.apmr.2017.06.020] [PMID]
- Rozenfeld E, Finestone A, Moran U, Damri E, Kalichman L. Test-retest reliability of myofascial trigger point detection in hip and thigh areas. Journal of Bodywork and Movement Therapies. 2017; 21(4):914-9. [DOI:10.1016/j.jbmt.2017.03.023]
- Licht G, Müller-Ehrenberg H, Mathis J, Berg G, Greitemann G. Untersuchung myofaszialer Triggerpunkte ist zuverlässig. Manuelle Medizin. 2007; 45(6):402-8. [DOI:10.1007/s00337-007-0559-0]
- Heitkamp H, Gärtner-Tschacher N, Schöttker-Königer T. Intertester reliability of myofacial trigger point palpation in the vastus medialis obliquus muscle. manuelletherapie. 2014; 18(05):227-35. [DOI:10.1055/s-0034-1396918]
- Mayoral Del Moral O, Torres Lacomba M, Russell IJ, Sánchez Méndez Ó, Sánchez Sánchez B. Validity and reliability of clinical examination in the diagnosis of myofascial pain syndrome and myofascial trigger points in upper quarter muscles. Pain Medicine. 2018; 19(10):2039-50. [DOI:10.1093/pm/pnx315] [PMID]
- De Groef A, Van Kampen M, Dieltjens E, De Geyter S, Vos L, De Vrieze T, et al. Identification of myofascial trigger points in breast cancer survivors with upper limb pain: Interrater reliability. Pain Medicine. 2018; 19(8):1650-6. [DOI:10.1093/pm/pnx299] [PMID]
- Dunning J, Butts R, Mourad F, Young I, Flannagan S, Perreault T. Dry needling: A literature review with implications for clinical practice guidelines. Physical Therapy Reviews. 2014; 19(4):252-65. [DOI:10.1179/108331913X13844245102034] [PMID]
- Kumbhare DA, Elzibak AH, Noseworthy MD. Assessment of myofascial trigger points using ultrasound. American Journal of Physical Medicine & Rehabilitation. 2016; 95(1):72-80. [DOI:10.1097/PHM.0000000000000376] [PMID]
- Adigozali H, Shadmehr A, Ebrahimi E, Rezasoltani A, Naderi F. Reliability of assessment of upper trapezius morphology, its mechanical properties and blood flow in female patients with myofascial pain syndrome using ultrasonography. Journal of Bodywork and Movement Therapies. 2017; 21(1):35-40. [DOI:10.1016/j.jbmt.2016.04.010] [PMID]
- Fernández-de-Las-Peñas C, Dommerholt J. International consensus on diagnostic criteria and clinical considerations of myofascial trigger points: A Delphi study. Pain Medicine. 2018; 19(1):142-50. [DOI:10.1093/pm/pnx207] [PMID]
- Sikdar S, Shah JP, Gebreab T, Yen RH, Gilliams E, Danoff J, et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Archives of Physical Medicine and Rehabilitation. 2009; 90(11):1829-38. [DOI:10.1016/j.apmr.2009.04.015] [PMID]
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: What is moderate pain in millimetres? Pain. 1997; 72(1-2):95-7. [DOI:10.1016/S0304-3959(97)00005-5] [PMID]
- Unverzagt C, Berglund K, Thomas J. Dry needling for myofascial trigger point pain: a clinical commentary. International Journal of Sports Physical Therapy. 2015; 10(3):402-18. [PMID]
- Dommerholt J, de las Penas CF. Trigger point dry needling E-book. Amsterdam: Elsevier Health Sciences; 2018. [Link]
- Faghri PD, Van Meerdervort HP, Glaser RM, Figoni SF. Electrical stimulation-induced contraction to reduce blood stasis during arthroplasty. IEEE Transactions on Rehabilitation Engineering. 1997; 5(1):62-9. [DOI:10.1109/86.559350] [PMID]
- Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983; 16(1):87-101. [DOI:10.1016/0304-3959(83)90088-X] [PMID]
- Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Academic Emergency Medicine. 2001; 8(12):1153-7. [DOI:10.1111/j.1553-2712.2001.tb01132.x] [PMID]
- Joyce CR, Zutshi DW, Hrubes V, Mason RM. Comparison of fixed interval and visual analogue scales for rating chronic pain. European Journal of Clinical Pharmacology. 1975; 8(6):415-20. [DOI:10.1007/BF00562315] [PMID]
- Reese NB, Bandy WD. Joint range of motion and muscle length testing-E-book. Amsterdam: Elsevier Health Sciences; 2016. [Link]
- Farooq MN, Mohseni Bandpei MA, Ali M, Khan GA. Reliability of the universal goniometer for assessing active cervical range of motion in asymptomatic healthy persons. Pakistan Journal of Medical Sciences. 2016; 32(2):457-61. [DOI:10.12669/pjms.322.8747] [PMID]
- Yamabata S, Shiraishi H, Munechika M, Fukushima H, Fukuoka Y, Hojo T, et al. Effects of electrical stimulation therapy on the blood flow in chronic critical limb ischemia patients following regenerative therapy. SAGE Open Medicine. 2016; 4:2050312116660723. [DOI:10.1177/2050312116660723] [PMID]
- Broderick BJ, O’Briain DE, Breen PP, Kearns SR, ÓLaighin G. A pilot evaluation of a neuromuscular electrical stimulation (NMES) based methodology for the prevention of venous stasis during bed rest. Medical Engineering & Physics. 2010; 32(4):349-55. [DOI:10.1016/j.medengphy.2010.01.006] [PMID]
- Griffin M, Nicolaides AN, Bond D, Geroulakos G, Kalodiki E. The efficacy of a new stimulation technology to increase venous flow and prevent venous stasis. European Journal of Vascular and Endovascular Surgery. 2010; 40(6):766-71. [DOI:10.1016/j.ejvs.2010.06.019] [PMID]
- DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Current Rheumatology Reports. 2008; 10(6):492-9. [DOI:10.1007/s11926-008-0080-z] [PMID]
- Ballyns JJ, Shah JP, Hammond J, Gebreab T, Gerber LH, Sikdar S. Objective sonographic measures for characterizing myofascial trigger points associated with cervical pain. Journal of Ultrasound in Medicine. 2011; 30(10):1331-40. [DOI:10.7863/jum.2011.30.10.1331] [PMID]
- Maher RM, Hayes DM, Shinohara M. Quantification of dry needling and posture effects on myofascial trigger points using ultrasound shear-wave elastography. Archives of Physical Medicine and Rehabilitation. 2013; 94(11):2146-50. [DOI:10.1016/j.apmr.2013.04.021] [PMID]
Type of Study: Original |
Subject:
Physical Therapy
Received: 12/10/2022 | Accepted: 14/02/2023 | Published: 1/10/2023
Received: 12/10/2022 | Accepted: 14/02/2023 | Published: 1/10/2023
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |